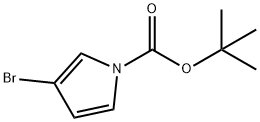
tert-Butyl 3-bromo-1H-pyrrole-1-carboxylate synthesis
- Product Name:tert-Butyl 3-bromo-1H-pyrrole-1-carboxylate
- CAS Number:475561-75-2
- Molecular formula:C9H12BrNO2
- Molecular Weight:246.1

24424-99-5
863 suppliers
$13.50/25G
87630-36-2
131 suppliers
$17.00/250mg

475561-75-2
13 suppliers
inquiry
Yield: 24%
Reaction Conditions:
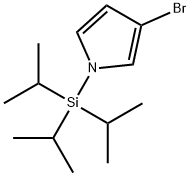
Stage #1:3-bromo-1-triisopropylsilanyl-1H-pyrrole with tetrabutyl ammonium fluoride in tetrahydrofuran at 20; for 0.5 h;
Stage #2:di-tert-butyl dicarbonate with dmap in tetrahydrofuran for 2 h;
Steps:
4
Preparation 4S-Bromo-pyrrole-l-carboxylic acid tert-butyl ester; The synthetic procedure described in this Preparation was carried out according to the process shown in Scheme E.SCHEME EA solution of TBAF (1.0 M in THF, 3.6 mL) was added to a solution of 3-bromo-l- triisopropylsilanyl-lH-pyrrole (1.0 g, 3.308 mmol) in TηF (10 mL) and the mixture was stirred at room temperature for 30 minutes. (BOC)2O (0.866 g, 3.965 mmol) and DMAP (40 mg, 0.3308 mmol) were added to the reaction and the resulting mixture was stirred for 2 additional hours. Water was added, and the mixture was extracted with EtOAc. The combined organic extracts were washed with water and with brine; dried OVCrNa2SO4, filtered, and evaporated under reduced pressure. The residue was purified via flash chromatography (hexane/EtOAc) to give 0.197 g (24% yield) of 3-bromo-pyrrole-l- carboxylic acid tert-butyl ester as a clear oil.
References:
F. HOFFMANN-LA ROCHE AG WO2008/55847, 2008, A1 Location in patent:Page/Page column 59

24424-99-5
863 suppliers
$13.50/25G
87630-40-8
49 suppliers
$435.00/250mg

475561-75-2
13 suppliers
inquiry