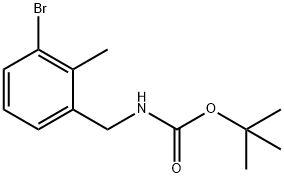
tert-butyl 3-bromo-2-methylbenzylcarbamate synthesis
- Product Name:tert-butyl 3-bromo-2-methylbenzylcarbamate
- CAS Number:1177558-64-3
- Molecular formula:C13H18BrNO2
- Molecular Weight:300.19
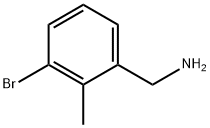
943722-02-9
34 suppliers
inquiry

24424-99-5
864 suppliers
$13.50/25G

1177558-64-3
6 suppliers
inquiry
Yield:1177558-64-3 35%
Reaction Conditions:
Stage #1: [(3-Bromo-2-methylphenyl)methyl]aminewith sodium carbonate in dichloromethane; for 0.25 h;
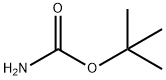
Stage #2: di-tert-butyl dicarbonate in dichloromethane;
Steps:
64 1,1-Dimethylethyl [(3-bromo-2-methylphenyl)methyl]carbamate
Example 64 1,1-Dimethylethyl [(3-bromo-2-methylphenyl)methyl]carbamate [(3-Bromo-2-methylphenyl)methyl]amine (4.0 g, 17.0 mmol) was suspended in CH2Cl2 (50 mL), then sodium carbonate (4.8 g, 45.3 mmol) was added. After stirred for 15 min, the solution of Boc2O (4.0 g, 18.3 mmol) in CH2Cl2 (20 mL) was added, and then the mixture was stirred overnight. After the solvent was removed, the residue was dissolved in CH2Cl2 (40 mL). The solution was washed with water (15 mL), brine (15 mL) and dried over anhydrous Na2SO4. After silica column chomatography, (eluted with petroleum ether: EtOAc=20:1 to 5:1), 1.3 g of the product, 1,1-dimethylethyl [(3-bromo-2-methylphenyl)methyl]carbamate, was obtained (yield: 35%). 1H NMR (400 MHz, CDCl3): δ 1.45 (s, 9H), 2.40 (s, 3H), 4.34 (d, J=6.0 Hz, 2H), 4.71 (br, 1H), 7.02 (t, J=8.0 Hz, 1H), 7.19 (d, J=7.6 Hz, 1H), 7.48 (dd, J=7.8 Hz, J=0.6 Hz, 1H).
References:
US2009/203657,2009,A1 Location in patent:Page/Page column 60
4248-19-5
379 suppliers
$5.00/5g
83647-40-9
117 suppliers
$10.00/100mg

1177558-64-3
6 suppliers
inquiry