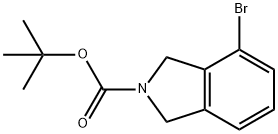
tert-butyl-4-bromoisoindoline-2-carboxylate synthesis
- Product Name:tert-butyl-4-bromoisoindoline-2-carboxylate
- CAS Number:1035235-27-8
- Molecular formula:C13H16BrNO2
- Molecular Weight:298.18
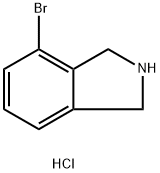
923590-95-8

24424-99-5

1035235-27-8
Step e. Over 5 min, triethylamine (TEA, 126 mL, 903 mmol) was added dropwise to a stirring suspension of 4-bromoisoindoline hydrochloride (100 g, 430 mmol) in tetrahydrofuran (THF, 700 mL) and the mixture was cooled to 10°C. The reaction temperature was controlled to be below 20°C. Subsequently, a solution of di-tert-butyl dicarbonate (122 g, 559 mmol) in THF (300 mL) was slowly added to the cooled mixture over a period of 15 min while the reaction temperature was controlled to be lower than 20 °C. The resulting mixture was stirred at room temperature for 12 hours. Upon completion of the reaction, the mixture was concentrated under vacuum to give an oil. The oily substance was partitioned between ethyl acetate (EtOAc, 600 mL) and water (600 mL). The aqueous phase was separated and extracted with EtOAc (2 x 500 mL). The organic extracts were combined, washed with brine, dried over anhydrous sodium sulfate (Na2SO4), filtered and evaporated to dryness in vacuum to give the crude product (161 g). The crude product was slurried in hexane (150 mL), filtered and the solid was washed with hexane (60 mL). Finally, it was dried under vacuum at room temperature to give tert-butyl 4-bromoisoindoline-2-carbonate (95.7 g, 75% yield).LCMS (Method R): retention time 3.02 min, m/z 242 (-tBu)/198 (-Boc) [M + H]+.NMR (400 MHz, DMSO-d6) δ ppm: 7.51-7.46 (dd. J = 7.8, 0.6 Hz, 1H), 7.38-7.30 (m, 1H), 7.29-7.21 (m, 1H), 4.73-4.63 (d, 2H), 4.56-4.47 (d, 2H), 1.50-1.42 (d, 9H).

923590-95-8
140 suppliers
$17.00/100mg

24424-99-5
871 suppliers
$13.50/25G

1035235-27-8
83 suppliers
$52.00/250mg
Yield:1035235-27-8 94%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 3 h;Inert atmosphere;
Steps:
Intermediate 4-bromoisoindoline-2-carboxylic acid benzyl ester
General procedure: 4-Bromoisoindoline hydrochloride (3.0 g, 13 mmol, 1 eq) was suspended in dry DCM (50 ml), and then TEA (5.4 ml, 39 mmol, 3 eq) was added. Reaction mixture was cooled to 0°C in an ice-bath, and then benzyl chloroformate (2.7 ml, 19 mmol, 1 .5 eq) dissolved in 10 ml DCM was added dropwise. Reaction was carried out under inert gas (nitrogen) atmosphere while stirring at room temperature for 3h. Reaction mixture was extracted thrice with saturated NaHCC solution, then once with water and with saturated NaCl solution. Organic phase was dried over anhydrous Na2S04, and then concentrated under reduced pressure to obtain white solid which was dried under vacuum. (0165) White solid, yield: 90% (3.8 g), Ci6Hi4BrN02, MW 332.19, monoisotopic mass 331 .02, [M+H]+ 332.2, UPLC Rt = 8.12. 1H NMR (300 MHz, CDCU) d (ppm) 4.73 (dd, J= 1 1 .6, 1 .4 Hz, 2H), 4.79-4.89 (m, 2H), 5.22 (d, J = 2.2 Hz, 2H), 7.09-7.50 (m, 7H).
References:
CELON PHARMA S.A.;UNIWERSYTET JAGIELLONSKI;INSTYTUT FARMAKOLOGII POLSKIEJ AKADEMII NAUK;KAMINSKI, Krzysztof;GRYCHOWSKA, Katarzyna;CANALE, Vittorio;BOJARSKI, Andrzej J.;SATALA, Grzegorz;LENDA, Tomasz;POPIK, Piotr;MATLOKA, Mikolaj;DUBIEL, Krzysztof;MOSZCZYNSKI-PETKOWSKI, Rafal;PIECZYKOLAN, Jerzy;WIECZOREK, Maciej;ZAJDEL, Pawel WO2019/97282, 2019, A1 Location in patent:Page/Page column 11; 12
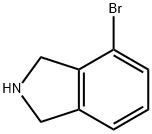
127168-81-4
71 suppliers
$55.00/1g

24424-99-5
871 suppliers
$13.50/25G

1035235-27-8
83 suppliers
$52.00/250mg