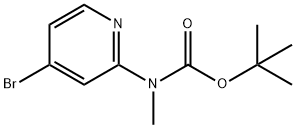
tert-Butyl (4-broMopyridin-2-yl)(Methyl)carbaMate synthesis
- Product Name:tert-Butyl (4-broMopyridin-2-yl)(Methyl)carbaMate
- CAS Number:946000-13-1
- Molecular formula:C11H15BrN2O2
- Molecular Weight:287.15
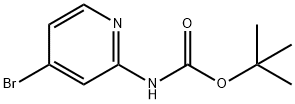
207799-10-8

74-88-4

946000-13-1
Step 1: Tert-butyl (4-bromopyridin-2-yl) carbamate (0.25 g, 0.915 mmol) was dissolved in DMF (5 ml) at 0 °C. To this solution, NaH (60% oil solution, 0.073 g, 1.83 mmol) was slowly added, keeping the reaction mixture stirred at 0°C for 15 min. Subsequently, methyl iodide (0.26 g, 1.37 mmol) was added at the same temperature. The reaction mixture was transferred to room temperature and stirring was continued for 1 hour. After completion of the reaction, the mixture was poured into ice-cold water (20 ml) and extracted with EtOAc (2 x 15 ml). The organic layers were combined, washed with brine (20 ml) and then dried over Na2SO4. After filtration, the organic phase was concentrated under reduced pressure to afford the crude product (tert-butyl (4-bromopyridin-2-yl)(methyl)carbamate (0.25 g, 0.87 mmol), which was used in the subsequent reaction without further purification.The LCMS analysis (Method A) showed a retention time of 2.50 min, and the mass spectra (MS: ES+) observed the peaks with m/z of 230.9 and 232.9 (M -56) ion peaks.

207799-10-8
142 suppliers
$11.00/250mg

74-88-4
357 suppliers
$15.00/10g

946000-13-1
16 suppliers
$71.00/50mg
Yield:946000-13-1 76%
Reaction Conditions:
Stage #1: (4-bromopyridin-2-yl)-carbamic acid tert-butyl esterwith sodium hydride in tetrahydrofuran at 0; for 0.25 h;
Stage #2: methyl iodine in tetrahydrofuran at 0 - 20; for 16 h;
Steps:
tert-Butyl (4-bromo-pyridin-2-yl)-methyl-carbamate (160)To a solution of te/7-butyl carbamate 155 (300 mg, 1.10 mmol) in THF (5 mL) at 0°C is added sodium hydride (53 mg, 1.32 mmol, 60% dispersion in mineral oil) in one portion. After stirring 15 minutes, iodomethane is added (82 μL, 1.32 mmol) and the reaction mixture warmed to RT and stirred 16 h. The reaction is then quenched with 5% citric acid (10 mL) and extracted into EtOAc (2 x 10 mL). The EPO
References:
WO2008/57497,2008,A2 Location in patent:Page/Page column 296-297
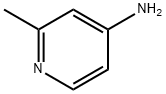
18437-58-6
326 suppliers
$6.00/1g

946000-13-1
16 suppliers
$71.00/50mg
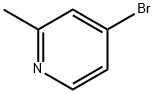
22282-99-1
405 suppliers
$6.00/5g

946000-13-1
16 suppliers
$71.00/50mg
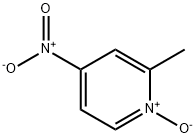
5470-66-6
302 suppliers
$8.00/1g

946000-13-1
16 suppliers
$71.00/50mg
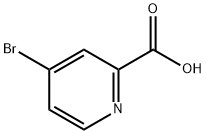
30766-03-1
271 suppliers
$7.00/1g

946000-13-1
16 suppliers
$71.00/50mg