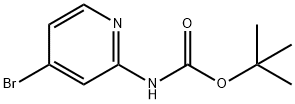
TERT-BUTYL 4-BROMOPYRIDIN-2-YLCARBAMATE synthesis
- Product Name:TERT-BUTYL 4-BROMOPYRIDIN-2-YLCARBAMATE
- CAS Number:207799-10-8
- Molecular formula:C10H13BrN2O2
- Molecular Weight:273.13
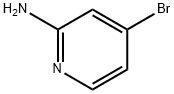
84249-14-9

24424-99-5

207799-10-8
General procedure for the synthesis of 2-Boc amine-4-bromopyridine from 2-amino-4-bromopyridine and di-tert-butyl dicarbonate: To a solution of 4-bromopyridin-2-amine (519 mg) in anhydrous THF (15 mL) was slowly added bis(trimethylmethylsilyl)amide (1 M solution in hexane, 6 mL) at -5 °C and the reaction mixture was stirred. After maintaining this temperature for 10 minutes, di-tert-butyl dicarbonate (654 mg) was added and the reaction mixture was transferred to room temperature to continue stirring for 1 hour. Upon completion of the reaction, the reaction was quenched by the addition of saturated ammonium chloride solution (20 mL) and the mixture was extracted with EtOAc. The organic layers were combined, washed sequentially with water and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to afford the target product 2-Boc amine-4-bromopyridine in 96% yield.
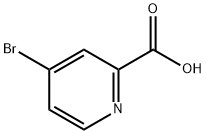
30766-03-1
271 suppliers
$7.00/1g

207799-10-8
142 suppliers
$11.00/250mg
Yield:207799-10-8 93%
Steps:
tert-Butyl (4-bromo-pyridin-2-yl)-carbamate (155)O HN^OT ohis c.omBrpound is prepared from 4-bromopyridine-2-carboxylic acid (154, 600 mg, 2.97 mmol) in accordance with the procedure of Deady, Korytsky and Rowe (Deady, L. W.; Korytsky, O. L.; Rowe, J. E. Aust. J. Chem., 1982, 35, 2025-2034). Yield: 750 mg (93%).
References:
CURIS, INC. WO2008/57497, 2008, A2 Location in patent:Page/Page column 293

84249-14-9
378 suppliers
$6.00/1g

24424-99-5
869 suppliers
$13.50/25G

207799-10-8
142 suppliers
$11.00/250mg

75-65-0
783 suppliers
$10.00/10ml

207799-10-8
142 suppliers
$11.00/250mg

30766-03-1
271 suppliers
$7.00/1g

75-65-0
783 suppliers
$10.00/10ml

207799-10-8
142 suppliers
$11.00/250mg
18437-58-6
326 suppliers
$6.00/1g

207799-10-8
142 suppliers
$11.00/250mg