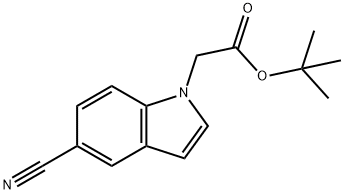
tert-butyl (5-cyano-1H-indol-1-yl)acetate synthesis
- Product Name:tert-butyl (5-cyano-1H-indol-1-yl)acetate
- CAS Number:1229608-56-3
- Molecular formula:C15H16N2O2
- Molecular Weight:256.3
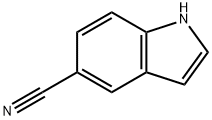
15861-24-2
454 suppliers
$10.00/5g
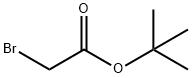
5292-43-3
456 suppliers
$10.00/5g

1229608-56-3
2 suppliers
inquiry
Yield:1229608-56-3 98%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 16 h;Reflux;
Steps:
1
tert-butyl {5-r(hvdroxyamino)(imino)methvH-1 H-indol-1 -yl)acetate Step 1: tert-butyl (5-cyano-1 H-indol-1 -yl)acetate tert-butyl bromoacetate (0.88 ml 5.99 mmol) was added to a suspension of 5-cyanoindole (0.71 g; 4.99 mmol) and K2CO3 (1.38 g; 9.99 mmol) in CH3CN (20 mL) and the resulting mixture was stirred at reflux for 16 hours. Filtration and concentration in vacuo gave a light yellow oil which crystallized upon standing. The solid was triturated in a mixture of Et2O and hexane to give the title compound as an off-white solid (1.26 g, 98%). HPLC (Method A), Rt: 4.43 min (Purity: 96.8%). LC/MS (Method B): 256.9 (M+H)+.
References:
WO2010/69949,2010,A1 Location in patent:Page/Page column 55