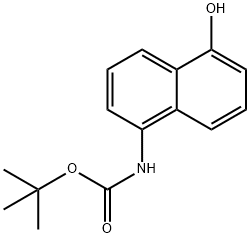
tert-butyl 5-hydroxynaphthalen-1-ylcarbamate synthesis
- Product Name:tert-butyl 5-hydroxynaphthalen-1-ylcarbamate
- CAS Number:848086-82-8
- Molecular formula:C15H17NO3
- Molecular Weight:259.3
83-55-6
181 suppliers
$40.00/250mg

24424-99-5
869 suppliers
$13.50/25G

848086-82-8
14 suppliers
inquiry
Yield:848086-82-8 96%
Reaction Conditions:
with Sodium hydrogenocarbonate in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 12 h;
Steps:
4.2.1. General method for Boc-protection of amino-1-naphthol (3-4)
General procedure: To a 100 mL round-bottom flask fitted with a magnetic stir bar wereadded amino-1-naphthol (1.59 g, 10.0 mmol), di-tert-butyl dicarbonate(2.40 g, 11.0 mmol), NaHCO3 (1.25 g, 15.0 mmol), THF (20 mL), andwater (10 mL). The mixture was stirred at room temperature and keptopen to air overnight. After completion of the reaction, THF wasremoved in vacuo and the resulting residue was diluted with water.Large amounts of black solids precipitated. The samples were thenfiltered and washed with water and hexane. The resulting black solidwas used for further purificationTert-butyl (5-hydroxynaphthalen-1-yl) carbamate (3). Black solid,yield 2.49 g (96%).1HNMR (500 MHz, CDCl3) d 7.96 (d, J8.3 Hz,d=1H), 7.91 (s, 1H), 7.45 (t, J = 9.0 Hz, 2H), 7.32 (t, J = 7.9 Hz, 1H), 6.81==(d, J6.7 Hz, 2H), 5.48 (s, 1H), 1.56 (s, 9H).13CNMR (126 MHz,=CDCl3) d 152.0, 132.8, 127.9, 126.0, 125.2, 117.9, 113.1, 108.8, 80.7,d28.4.
References:
Sun, Hongshun;Du, Yijing;Chen, Xu;Jiang, Hong;Li, Yulong;Shen, Linjiang [Dyes and Pigments,2022,vol. 208,art. no. 110804]