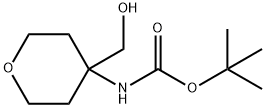
tert-butyl N-[4-(hydroxymethyl)oxan-4-yl]carbamate synthesis
- Product Name:tert-butyl N-[4-(hydroxymethyl)oxan-4-yl]carbamate
- CAS Number:1029716-09-3
- Molecular formula:C11H21NO4
- Molecular Weight:231.29
720706-20-7
79 suppliers
$84.00/100mg

24424-99-5
869 suppliers
$13.50/25G
![tert-butyl N-[4-(hydroxymethyl)oxan-4-yl]carbamate](/CAS/20180703/GIF/1029716-09-3.gif)
1029716-09-3
35 suppliers
$110.00/50mg
Yield:1029716-09-3 74%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 70; for 5 h;Inert atmosphere;
Steps:
26 tert-Butyl (4-(hydroxymethyl)tetrahydro-2H-pyran-4-yl)carbamate
Di-tert-butyl dicarbonate (6.39 g, 29.27 mmol) was added to triethylamine (3.70 g, 36.59mmol), (4-aminotetrahydro-2H-pyran-4-yl)methanol (3.2 g, 24.40 mmol) in THF (40 mL)under nitrogen. The resulting mixture was stirred at 70 °C for 5 hours. The reactionmixture was quenched with water (50 mL), extracted with EtOAc (3 x 50 mL), the organic layer was then dried over Na2SO4, filtered and evaporated to afford yellow oil. The crude product was purified by flash silica chromatography, elution gradient 10 to 30% EtOAc in petroleum ether. Pure fractions were evaporated to dryness to afford tert-butyl (4- (hydroxymethyl)tetrahydro-2H-pyran-4-yl)carbamate (4.20 g, 74%) as a white solid. ‘HNMR (300 MHz, DMSO, 30°C) 1.45 (9H, s), 1.50-1.84 (2H, m), 3.31-3.61 (6H ,m), 4.61- 4.66 (1H, m), 6.36 (1H, s). mlz (ES+), [M+H]+ = 232.
References:
WO2016/162325,2016,A1 Location in patent:Page/Page column 197
172843-97-9
105 suppliers
$29.00/100mg
![tert-butyl N-[4-(hydroxymethyl)oxan-4-yl]carbamate](/CAS/20180703/GIF/1029716-09-3.gif)
1029716-09-3
35 suppliers
$110.00/50mg