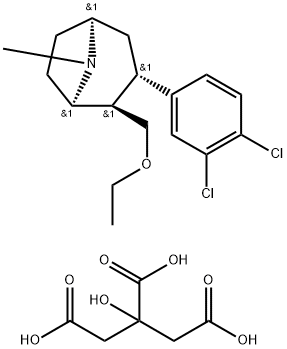
Tesofensine Intermediate 1 synthesis
- Product Name:Tesofensine Intermediate 1
- CAS Number:173667-94-2
- Molecular formula:C15H19Cl2NO
- Molecular Weight:300.22
Yield:861205-83-6 83.85%
Reaction Conditions:
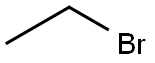
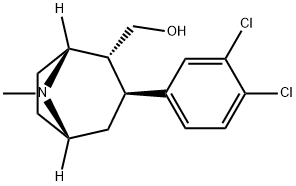
Stage #1: ethyl bromide;(1R,2R,3S)-2-hydroxymethyl-3-(3,4-dichlorophenyl)-tropanewith potassium hydroxide;tetra(n-butyl)ammonium hydrogensulfate in 1,2-dimethoxyethane at 20 - 62; for 2.75 h;
Stage #2: in methanol;acetone at 15 - 55; for 1 h;Product distribution / selectivity;
Steps:
2
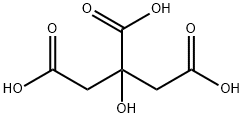
EXAMPLE 2; (1R,2R,3S)-2-ethoxymethyl-3-(3,4-dichlorophenyl)-tropane citrate; 17.5 g (0.161 mol) of ethyl bromide dissolved in 20 ml of 1,2-dimethoxyethane are metered, within 15 minutes, into a mixture of 12 g (0.0400 mol) of (1R,2R,3S)-2-hydroxymethyl-3-(3,4-dichlorophenyl)-tropane (prepared according to WO9730997), 17.9 g of powdered (0.320 mol KOH) caustic potash, 1.39 g (0.00409 mol) of tetra-n-butylammonium hydrogen sulphate, and 220 ml DME at a temperature between 20 and 31° C., with stirring. After the addition has ended, the mixture is stirred for 1.5 hours at a temperature between 58 and 62° C. Then 76 ml of water are added, the mixture is stirred for another hour at this temperature, and the organic phase is separated off. The organic phase is evaporated down under reduced pressure using the rotary evaporator. The residue is dissolved with 108 ml acetone at 55° C., filtered and rinsed with 40 ml acetone. The solution obtained is treated with a mixture of 7.68 g (0.0400 mol) of citric acid and 24 ml of methanol at 40° C. The crystal suspension is cooled to 20° C. and stirred for one hour at 15 to 20° C. The crystals obtained are isolated and washed with at least 80 ml acetone. After drying in the vacuum drying cupboard at 40° C. 17.44 g (83.85% of theory) of the title compound are obtained as yellowish crystals with a purity of more than 99.5%.
References:
US2005/171145,2005,A1 Location in patent:Page/Page column 2