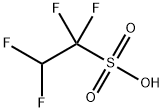
TETRAFLUOROETHANESULFONIC ACID synthesis
- Product Name:TETRAFLUOROETHANESULFONIC ACID
- CAS Number:464-14-2
- Molecular formula:C2H2F4O3S
- Molecular Weight:182.09
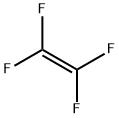
116-14-3
53 suppliers
inquiry

464-14-2
15 suppliers
$844.07/5G
Yield:464-14-2 69 g
Reaction Conditions:
Stage #1: polytetrafluoroethylenewith sodium sulfite in water at -130 - 120; for 12 h;
Stage #2: with hydrogenchloride in methanol at 15; for 1.25 h;
Steps:
1b Preparation of Tetrafluoroethanesulphonic Acid
The sodium sulphite (201 g) was dissolved in water (600 mL) and taken in a 1000 mL Hastelloy reactor. Tetrafluoroethylene (150 g) was added to the reactor at -130°C. The temperature of the reactor was increased to 120°C and maintained at 120°C for 12 hours. The reactor was cooled to 25°C and unreacted tetrafluoroethylene was vented off. The water of the reaction was removed from reaction mixture by azeotropic distillation to obtain a reaction mass. The reaction mass was extracted with hot ethanol using soxhlet apparatus. The ethanolic solution was concentrated to obtain pure sodium tetrafluoroethane sulfonate (100 g). The sodium tetrafluoroethane sulfonate (100 g) and methanol (140 g) were taken in a reaction vessel and the mixture was cooled to 15°C. The dry hydrochloric acid (100 g, 35%) was passed through the mixture for 45 minutes. The mixture was further stirred for 30 minutes, filtered and washed with methanol (15 g). The filtrate (244 g) was added to water (125 g) and mixture was distilled using one foot column. The distillate (319 g) was obtained at 60°C/10 mm Hg. Yield: 96 g (Moisture content: 23.30%; Tetrafluoroethanesulphonic acid - 72.3%) The tetrafluoroethanesulfonic acid monohydrate (96 g) was taken in a reaction vessel and thionyl chloride (160 g) was added drop-wise at 25°C over a period of 3 hours. The temperature of reaction mixture was raised to 80°C and the mixture was refluxed for 30 minutes. The mixture was cooled to 40°C. The crude material (69 g) was subjected to distillation under vacuum. Yield: 64 g Purity (%): 98.5
References:
WO2014/20614,2014,A2 Location in patent:Page/Page column 4; 5; 6