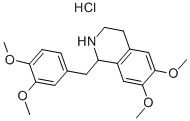
Tetrahydropapaverine hydrochloride synthesis
- Product Name:Tetrahydropapaverine hydrochloride
- CAS Number:6429-04-5
- Molecular formula:C20H25NO4.HCl
- Molecular Weight:379.8814
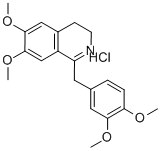
5884-22-0

6429-04-5
Crude 3,4-dihydroisoquinoline hydrochloride 12 (6.48 g) was suspended in methanol (50 mL) under argon protection and cooled to 0 °C. Sodium borohydride (0.8 g, 21 mmol) was slowly added to this suspension. The reaction mixture was stirred at room temperature for 10 minutes and then concentrated. Subsequently, brine (16 mL) and acetonitrile (30 mL) were added to the concentrate and the mixture was then partitioned between ethyl acetate (30 mL) and water (30 mL). The aqueous layer was extracted twice with a solvent mixture of ethyl acetate-acetonitrile (30 mL, 1:1). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate and concentrated to give a yellow oil. This oily substance was dissolved in methanol (10 mL), 5% hydrochloric acid solution in methanol (20 mL) was added and concentrated again. The residue was crystallized by the addition of ether, and the solid was collected by filtration and washed with ether to give finally 1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (6-HCl, 4.67 g, 89% yield, after a 3-step reaction) as a white solid with a melting point of 211-215 °C. The 1H NMR (DMSO-d6) data were as follows: δ 2.91 (1H, m), 3.16-3.24 (3H, m), 3.36 (1H, m), 3.56 (1H, m), 3.60 (3H, s), 3.80 (3H, s), 3.85 (6H, s), 4.73 (1H, m), 6.17 (1H, s), 6.58 ( 1H, s), 6.72 (1H, d, J = 8.0 Hz), 6.77-6.79 (2H, m), 9.77 (1H, br s), 10.35 (1H, br s). The 1H NMR (D2O) data were as follows: δ 3.01-3.05 (2H, m), 3.16-3.29 (2H, m), 3.41 (1H, m), 3.53 (1H, m), 3.60 (3H, s), 3.72 (3H, s), 3.83 (6H, s), 4.73 (1H, m), 6.36 (1H, s), 6.73 ( 1H, s), 6.83-6.89 (2H, m), 7.00 (1H, m).

5884-22-0
81 suppliers
inquiry

6429-04-5
262 suppliers
$10.00/1g
Yield:6429-04-5 89%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0 - 20; for 0.166667 h;Inert atmosphere;
Steps:
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (6?HCl)
To a stirred suspension of crude 3,4-dihydroisoquinoline hydrochloride 12 (6.48 g) in MeOH (50 mL) wasslowly added NaBH4 (0.8 g, 21 mmol) at 0 °C under Ar. The mixture was stirred for 10 min at roomtemperature and then concentrated. After an addition of brine (16 mL) and MeCN (30 mL), the resultingmixture was partitioned between AcOEt (30 mL) and H2O (30 mL). The aqueous layer was extracted twicewith AcOEt-MeCN (30 mL, 1:1). The combined organic layers were washed with brine, dried over Na2SO4and concentrated to give yellow oil, which was dissolved in MeOH (10 mL). HCl in MeOH (5% solution,20 mL) was added to the solution, and then concentrated. The residue was crystallized by an addition ofEt2O, and the solids were filtered and washed with Et2O to give 6?HCl (4.67 g, 89% for 3 steps) as whitesolids of mp 211-215 °C. 1H NMR: 2.91 (1H, m), 3.16-3.24 (3H, m), 3.36 (1H, m), 3.56 (1H, m), 3.60 (3H, s), 3.80 (3H, s), 3.85 (6H,1316 HETEROCYCLES, Vol. 88, No. 2, 2014s), 4.73 (1H, m), 6.17 (1H, s), 6.58 (1H, s), 6.72 (1H, d, J = 8.0), 6.77-6.79 (2H, m), 9.77 (1H, br s), 10.35(1H, br s).1H NMR (D2O):9b 3.01-3.05 (2H, m), 3.16-3.29 (2H, m), 3.41 (1H, m), 3.53 (1H, m), 3.60 (3H, s), 3.72 (3H,s), 3.83 (6H, s), 4.73 (1H, m), 6.36 (1H, s), 6.73 (1H, s), 6.83-6.89 (2H, m), 7.00 (1H, m).
References:
Yamamoto, Yasutomo;Tabuchi, Yuri;Baba, Ayana;Hideshima, Kumiko;Nakano, Mai;Miyawaki, Akari;Tomioka, Kiyoshi [Heterocycles,2014,vol. 8,# 2,p. 1311 - 1321]
![1-[(3,4-Dimethoxyphenyl)methyl]-3,4-dihydro-6,7-dimethoxyisoquinoline](/CAS/GIF/6957-27-3.gif)
6957-27-3
57 suppliers
inquiry

6429-04-5
262 suppliers
$10.00/1g
93-40-3
472 suppliers
$10.00/5g

6429-04-5
262 suppliers
$10.00/1g
13074-31-2
56 suppliers
$265.00/1g

6429-04-5
262 suppliers
$10.00/1g
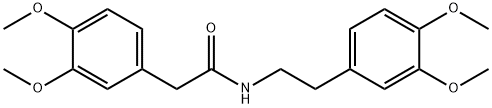
139-76-4
39 suppliers
inquiry

6429-04-5
262 suppliers
$10.00/1g