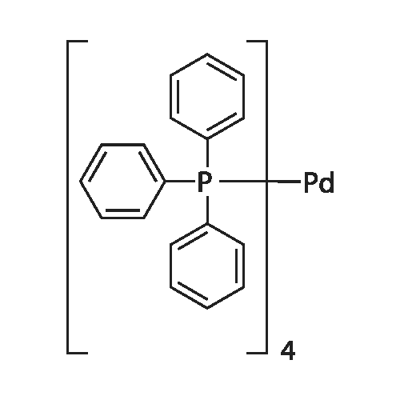
Tetrakis(triphenylphosphine)palladium synthesis
- Product Name:Tetrakis(triphenylphosphine)palladium
- CAS Number:14221-01-3
- Molecular formula:C72H60P4Pd
- Molecular Weight:1155.562
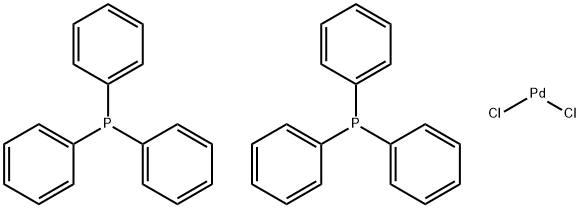
13965-03-2
577 suppliers
$20.00/1g

14221-01-3
648 suppliers
$22.00/1g
Yield:14221-01-3 98.48%
Reaction Conditions:
with formic acid in tetrahydrofuran at 63 - 100;Inert atmosphere;Solvent;
Steps:
1.2-1.5; 2; 3.2-3.5; 4-5
in the reactor B, add formic acid and heat it to boiling; blow nitrogen gas into the lower part of the liquid level of the reactor B, the nitrogen gas containing the formic acid vapor is led out above the reactor B, and the temperature of the gas is heated to 100 through the heat exchanger ; in the tower reactor C, add 5L isopropyl ether, heat to the liquid surface temperature (63±3) ; the solution obtained in step 1 is added dropwise from the top of the reactor C, and at the same time bubbling under the liquid surface In step 2, the nitrogen containing formic acid vapor derived from the reactor B; a reflux device is provided above the reactor C, and the exhaust gas that cannot be condensed is discharged; the formic acid contained in the exhaust gas that is discharged is condensed and recovered; when observing more precipitation in the reactor C, open the bottom valve and slowly release the liquid phase 1L in the lower part of the reactor, and the precipitation flows out accordingly; the liquid phase released in step 4 is cooled to below 30°C and then introduced into filter device D, and the product tetrakis(triphenylphosphine)palladium is filtered out, dried and packaged after filtration; the filtrate is recovered and put back into reactor C. It should be noted that steps 4 and 5 were repeated for a total of 3 times when more precipitation appeared in the reactor. 30 minutes after the solution in reactor A was added to reactor C, the heating was stopped, and 0.3 mol of formic acid was consumed in reactor B in total. After the device was cooled to room temperature, the whole solution of reactor C was released in batches, filtered, dried and packaged. Filtration was carried out by vacuum filtration, and drying was carried out by introducing nitrogen gas at 80°C and vacuuming under the filter element for 5 minutes.Finally, the obtained products were combined and weighed to obtain 113.8 g of yellow-green crystals with a yield of 98.48%
References:
CN114933612,2022,A Location in patent:Paragraph 0037-0049

13965-03-2
577 suppliers
$20.00/1g
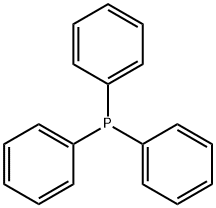
603-35-0
733 suppliers
$5.00/5G

14221-01-3
648 suppliers
$22.00/1g
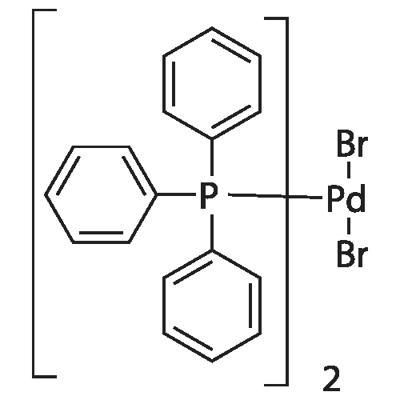
22180-53-6
44 suppliers
$23.00/250mg

603-35-0
733 suppliers
$5.00/5G

14221-01-3
648 suppliers
$22.00/1g
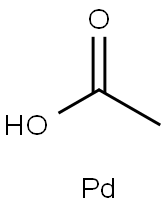
3375-31-3
554 suppliers
$23.00/100mg

603-35-0
733 suppliers
$5.00/5G

14221-01-3
648 suppliers
$22.00/1g
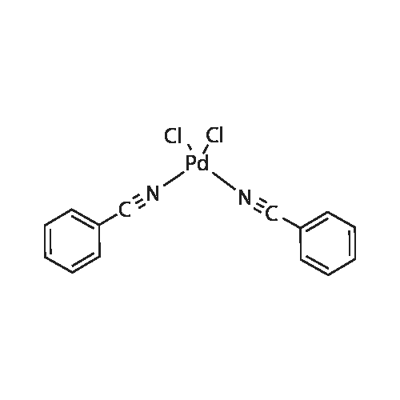
14220-64-5
344 suppliers
$13.00/100mg

14221-01-3
648 suppliers
$22.00/1g