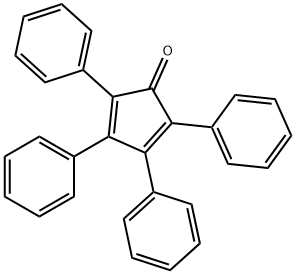
Tetraphenylcyclopentadienone synthesis
- Product Name:Tetraphenylcyclopentadienone
- CAS Number:479-33-4
- Molecular formula:C29H20O
- Molecular Weight:384.47
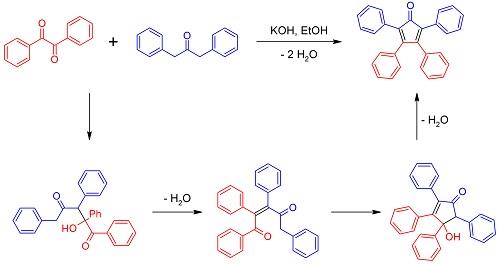
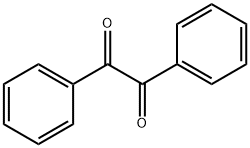
Tetraphenylcyclopentadienone has been prepared by the action of phenylmagnesium bromide on benzaldiphenylmaleide, and by reduction, dehydration, and oxidation of the methylenedesoxybenzoin obtained by condensing formaldehyde with desoxybenzoin. The present procedure is essentially that of Dilthey.
Synthesis of tetraphenylcyclopentadienone
Yield:479-33-4 90%
Reaction Conditions:
with potassium hydroxide in ethanol for 2 h;Reflux;
Steps:
2.1 2.2.1 Synthesis of tetraphenylcyclopentadienone (1)
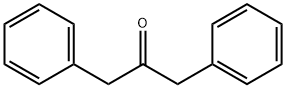
Benzil (6.3g, 0.03mol) and 1,3-dibenzyl ketone (6.3g, 0.03mol) were dissolved in 60mL of ethanol and then heated with stirring. When the mixture started to reflux, KOH (0.8g dissolved in 8mL of ethanol) was dropped into the mixture and allowed to react for 2h until black precipitate formed. After the solution was cooled to room temperature, the precipitate was filtered and washed with ethanol for three times to obtain a black solid 17 product with yield of 90% . Melt point: 219°C. 1H NMR (CDCl3, 400MHz, ppm): δ 8.08 (d, 2H), 7.86 (m, 6H), 7.61 (dd, 2H), 7.54 (dd, 4H), 7.42 (t, 2H). 13C NMR (CDCl3, 100MHz, ppm): δ 154.2, 132.1, 131.5, 131.4, 129.0, 128.6, 128.3, 127.8, 121.7, 120.9. ESI-MS spectrometry: m/z calculated for C29H20O [M+H]+: 384.15; found: 384.16. Anal. Calcd for C29H20O: C 90.60%, H 4.16%; found: C 90.66%, H 4.17%.
References:
Liang, Yan [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2019,vol. 216,p. 395 - 403]

79-37-8
490 suppliers
$17.67/10gm:
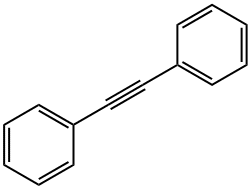
501-65-5
220 suppliers
$16.00/5g

479-33-4
204 suppliers
$11.00/1g

501-65-5
220 suppliers
$16.00/5g
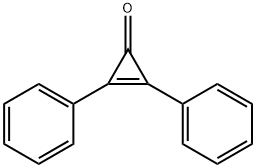
886-38-4
195 suppliers
$26.00/500mg

479-33-4
204 suppliers
$11.00/1g
201230-82-2
1 suppliers
inquiry

501-65-5
220 suppliers
$16.00/5g
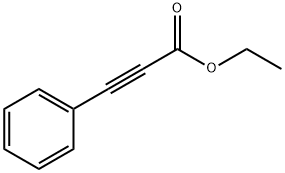
2216-94-6
162 suppliers
$12.00/1g

479-33-4
204 suppliers
$11.00/1g