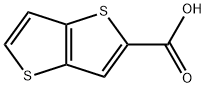
THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID synthesis
- Product Name:THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID
- CAS Number:1723-27-9
- Molecular formula:C7H4O2S2
- Molecular Weight:184.24
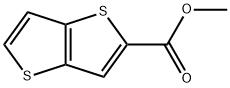
![Thieno[3,2-b]thiophene-2-carboxylic acid ethyl ester](/CAS2/GIF/201004-08-2.gif)
201004-08-2
![THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/1723-27-9.gif)
1723-27-9
Lithium hydroxide monohydrate (17.8 kg, 662.4 mol, 1.47 eq.) with deionized water (440.0 kg) was added to a 2000 L autoclave under a nitrogen atmosphere and stirred until completely dissolved. Subsequently, ethyl thieno[3,2-b]thiophene-2-carboxylate (95.5 kg, 449.8 mol, 1.0 eq.) was pumped into the reaction mixture at room temperature and tetrahydrofuran (379.5 kg) was added. The reaction mixture was heated to reflux (about 100°C) and maintained for 3 hours. Upon completion of the reaction, it was cooled to 35-40°C and the tetrahydrofuran was removed by rotary evaporation under reduced pressure under nitrogen atmosphere. Deionized water (200 kg) was added to the reaction vessel and the temperature was controlled to be below 20°C. The reaction mixture was filtered and the filter cake was washed three times with cold water (20 kg) and the residual liquid was removed by centrifugation to give the final thieno[3,2-b]thiophene-2-carboxylic acid (146 kg, 100% yield).
![Thieno[3,2-b]thiophene-2-carboxylic acid ethyl ester](/CAS2/GIF/201004-08-2.gif)
201004-08-2
42 suppliers
$77.76/250mgs:
![THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/1723-27-9.gif)
1723-27-9
134 suppliers
$21.00/100mg
Yield:1723-27-9 100%
Reaction Conditions:
with lithium hydroxyde monohydrate in lithium hydroxide monohydrate at 100; for 3 h;Inert atmosphere;Large scale;
Steps:
1.3
Under a nitrogen atmosphere, lithium hydroxide monohydrate (17. 8 Kg, 662.4 mol, 1.47 eq), H20 (440.0 OKg) was added to the 2000 L autoclave and stirred to dissolve the lithium hydroxide monohydrate in water. At room temperature, the intermediate 3 (95.5 ?, 449.8111 01, 1.069) was pumped into the reaction mixture, followed by 1 '(379.5 ). Heating to reflux (about 100 ° C) for 3 hours, cooling to 35 to 40 ° C and removing the tetrahydrofuran by rotary evaporation under reduced pressure under a nitrogen atmosphere Water (200 Kg) was added to the reaction vessel Controlled temperature below 20 ° C (74.6 Kg), filtered and the filter cake was washed three times with cold water (20 Kg) and centrifuged until no liquid was obtained, product 4 (146 Kg, yield 100%)..
References:
CN103172646,2016,B Location in patent:Paragraph 0108-0110
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
276 suppliers
$8.00/250mg

124-38-9
134 suppliers
$214.00/14L
![THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/1723-27-9.gif)
1723-27-9
134 suppliers
$21.00/100mg
98800-10-3
68 suppliers
$75.00/100mg
![THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/1723-27-9.gif)
1723-27-9
134 suppliers
$21.00/100mg
![Thieno[3,2-b]thiophene-2-carboxaldehyde](/CAS/GIF/31486-86-9.gif)
31486-86-9
111 suppliers
$45.00/50mg
![THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/1723-27-9.gif)
1723-27-9
134 suppliers
$21.00/100mg
930-96-1
242 suppliers
$11.00/1g
![THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/1723-27-9.gif)
1723-27-9
134 suppliers
$21.00/100mg