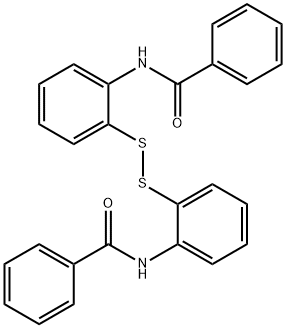
Thiobenzoic acid S-[2-(benzoylamino)phenyl] ester synthesis
- Product Name:Thiobenzoic acid S-[2-(benzoylamino)phenyl] ester
- CAS Number:1047-61-6
- Molecular formula:C20H15NO2S
- Molecular Weight:333.4
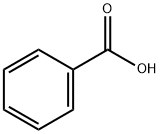
65-85-0
1332 suppliers
$10.00/25g
1141-88-4
201 suppliers
$8.00/1g
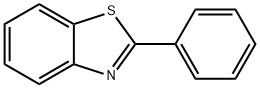
883-93-2
139 suppliers
$8.00/1g
![Thiobenzoic acid S-[2-(benzoylamino)phenyl] ester](/CAS/GIF/1047-61-6.gif)
1047-61-6
2 suppliers
inquiry
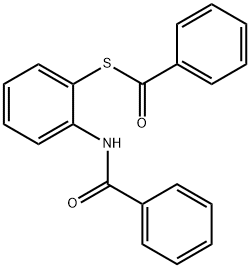
Yield:883-93-2 67% ,1047-61-6 11%
Reaction Conditions:
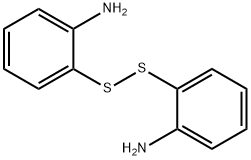
Stage #1: benzoic acid;2-Aminophenyl disulfide in toluene at 70; for 2.5 h;Inert atmosphere;
Stage #2: with phosphorus trichloride in toluene at 30 - 100;Inert atmosphere;
Steps:
General procedure for the synthesis of benzothiazole derivatives 3a-3p, D.
General procedure: 2,2'-Disulfanediyldianiline 1a or 6,6'-disulfanediylbis(3-chloroaniline) 1b (0.5 mmol) and carboxylic acid (1.0 mmol, except 0.5mmol for 2k) were added to a three-neck flask under an atmosphere of Ar, and 5 mL dry toluene was added, then the mixture was stirred for 2.5 h at 70 °C to allow the solid to dissolve in toluene solvent completely. After the reaction solution was cooled down to 30 °C, PCl3 (1.2 mmol, 0.165 g) was added dropwise. When the PCl3 was added completely, the reaction mixture was further stirred for 4 - 6 h at 100°C until no 2,2'-disulfanediyldianiline or 6,6'-disulfanediylbis(3-chloroaniline) was detected by TLC analysis. The reaction solution was washed with saturated aqueous sodium bicarbonate solution and extracted with CH2Cl2. The collected organic layers were dried over anhydrous MgSO4. After filtered toremove the MgSO4 and the solvent was removed under reduced pressure. The crude product was purifiedby flash chromatography on silica gel using PE / EtOAc.
References:
Du, Guangyan;Zhu, Ning;Han, Limin;Hong, Hailong;Suo, Quanling [Heterocycles,2015,vol. 91,# 9,p. 1723 - 1734]
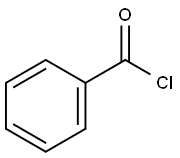
98-88-4
571 suppliers
$10.00/5g
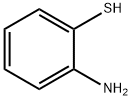
137-07-5
289 suppliers
$10.00/5g

883-93-2
139 suppliers
$8.00/1g
![Thiobenzoic acid S-[2-(benzoylamino)phenyl] ester](/CAS/GIF/1047-61-6.gif)
1047-61-6
2 suppliers
inquiry

3335-22-6
2 suppliers
inquiry
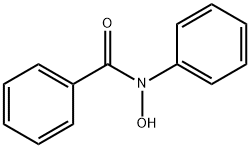
304-88-1
171 suppliers
$5.00/250mg
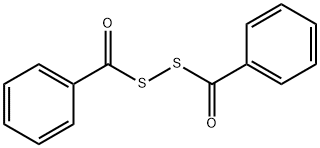
644-32-6
18 suppliers
$172.00/1g
![Thiobenzoic acid S-[2-(benzoylamino)phenyl] ester](/CAS/GIF/1047-61-6.gif)
1047-61-6
2 suppliers
inquiry
![Benzenecarbothioic acid, S-[4-(benzoylamino)phenyl] ester](/CAS/20210305/GIF/89567-88-4.gif)
89567-88-4
0 suppliers
inquiry