(+)-Fluprostenol synthesis
- Product Name:(+)-Fluprostenol
- CAS Number:54276-17-4
- Molecular formula:C23H29F3O6
- Molecular Weight:458.47
![2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-](/CAS/20180703/GIF/1204185-88-5.gif)
1204185-88-5
20 suppliers
inquiry
17814-85-6
410 suppliers
$8.00/5g

54276-17-4
68 suppliers
$51.00/1mg
Yield: 463 g
Reaction Conditions:
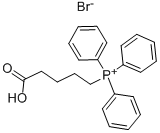
Stage #1:(4-carboxybutyl)triphenylphosphonium bromide with potassium tert-butylate in tetrahydrofuran at 0; for 0.25 h;Inert atmosphere;Large scale;Wittig Olefination;
Stage #2:(3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-2,5-diol in tetrahydrofuran at -10;Inert atmosphere;Large scale;Wittig Olefination;
Steps:
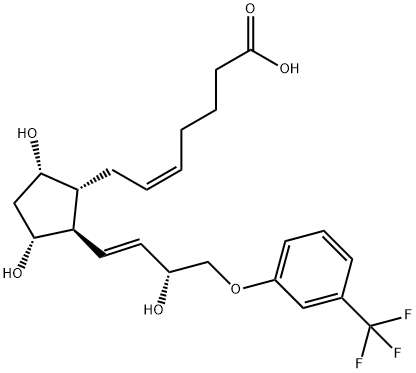
5 5. Construction of the upper chain (Preparation of Travoprost acid) Preparation of 5-heptanoic acid, 7-[(lR,2R,3R,5S)-3,5-dihydroxy-2-[(lE,3R)-3-hydroxy-4- [3-(trifluoromethyl)phenoxy]- 1 -buten- 1 -yljcyclopentyl]-, (5Z)- / Compound of formula (VI) /
Travoprost 4. intermediate Travoprost 5. intermediate (triol) (acid) CisH2iF305 C23H29F306 Mr: 374.36 Mr: 458.48 Under nitrogen atmosphere 1509 g of 4-carboxybutyl-phosphonium bromide (KBFBr) is dissolved in 12.8 L of THF, the solution is cooled to 0°C, and by maintaining that temperature, 1.12 kg of potassium tert-butylate is added to it in portions. After 15 minutes of stirring the reaction mixture is cooled to (-)10°C, then 367 g of triol dissolved in 2.24 L of THF is added and the mixture is stirred at (-10)°C. When the reaction has completed, the reaction mixture is decomposed with water and toluene is added. The aqueous phase is extracted with dichloromethane (DKM) and acidified with a solution of NaHS04. Ethyl acetate is then added, the phases are separated and the aqueous phase is extracted with ethyl acetate. The united organic phase is washed with a diluted sodium chloride solution, dried over Na2S04, the drying material is filtered off, the filtrate is washed and the filtrate solution is evaporated. The residue is crystallized from acetone : diisopropyl ether mixture. The crystals are filtered off, washed with diisopropyl ether : acetone mixture. The mother liquor is evaporated. Yield: 463 g, 103% IR spectrum of Travoprost 5. intermediate is shown on Figure 6. Travoprost 5. intermediate , l3C and l9F NMR data: : Over apping H NMR signals.
References:
CHINOIN GYÓGYSZER ÉS VEGYÉSZETI TERMÉKEK GYÁRA ZRT.;KARDOS, Zsuzsanna;KISS, Tibor;LÁSZLOFI, István;HORTOBÁGYI, Irén;BISCHOF, Zoltán;BÓDIS, Ádám;HAVASI, Gábor WO2013/93528, 2013, A1 Location in patent:Page/Page column 22-25
955005-75-1
1 suppliers
inquiry

54276-17-4
68 suppliers
$51.00/1mg
64091-14-1
23 suppliers
$165.00/500mg

54276-17-4
68 suppliers
$51.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

54276-17-4
68 suppliers
$51.00/1mg
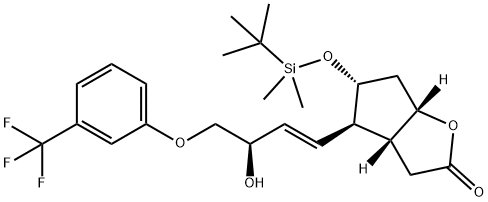
1240483-28-6
3 suppliers
inquiry

54276-17-4
68 suppliers
$51.00/1mg