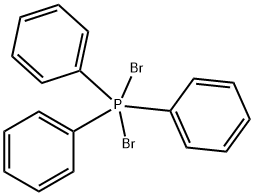
TRIPHENYLPHOSPHINE DIBROMIDE synthesis
- Product Name:TRIPHENYLPHOSPHINE DIBROMIDE
- CAS Number:1034-39-5
- Molecular formula:C18H15Br2P
- Molecular Weight:422.09
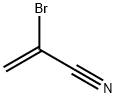
791-28-6
535 suppliers
$6.00/25g

1034-39-5
162 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
with bromine in acetonitrile at 0 - 5;
Steps:
22 Embodiment 22
Embodiment 22
27.8 g (100 mmol) of triphenylphosphine oxide was dissolved in 150 ml acetonitrile and the solution was cooled to 0?5° C., and 16.5 g (102 mmol) of bromine was slowly added to the cooled solution under stirring to obtain a reaction solution, and then the reaction solution was still stirred for 15 mins to obtain a solution of dibromo triphenylphosphine oxide. 44.4 g (98 mmol) of the disubstituted urea obtained in embodiment 11 and 20.2 g (200 mmol) triethylamine were suspended in 50 ml acetonitrile, the suspension was slowly added to the above solution of dibromo triphenylphosphine oxide in the temperature of 0-5° C., then the reaction was still carried out for 0.5-5 hours.
After the reaction was completed, 200 ml water was added and the organic phase was extracted by 500 ml of ethyl acetate, and then the solution containing organic phase was condensed to 50 ml and next 100 ml isopropanol was added, subsequently 33 ml water was dropwise added in the temperature of 40-50° C., then the final solution was cooled to room temperature and stirred for 2-5 hours, followed by filtered and dried to obtain 31 g of N-(4-oxyphenyl)-6-methyl-p-toluenesulfonyl-1H-benzimidazolyl-2-amine, the yield was 77%.
1H NMR (400 MHz, CDCl3): δ 8.41 (br, 1H), 7.75-7.79 (m, 2H), 7.56-7.61 (m, 3H), 7.18-7.25 (m, 3H), 6.89-6.92 (m, 3H), 3.99-4.04 (m, 2H), 2.35 (s, 3H), 2.31-2.33 (m, 3H), 1.40 (t, J=11.2 Hz, 3H).
References:
US2013/345436,2013,A1 Location in patent:Paragraph 0085; 0086; 0087
603-35-0
733 suppliers
$5.00/5G

1034-39-5
162 suppliers
$10.00/1g
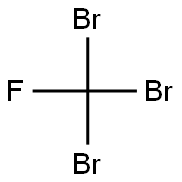
353-54-8
106 suppliers
$51.00/5g

603-35-0
733 suppliers
$5.00/5G
![Phosphonium, [fluoro(triphenylphosphoranylidene)methyl]triphenyl-, bromide (1:1)](/CAS/20211123/GIF/88410-12-2.gif)
88410-12-2
0 suppliers
inquiry

1034-39-5
162 suppliers
$10.00/1g

112-31-2
418 suppliers
$6.00/10g
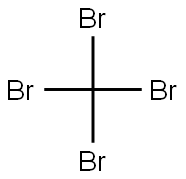
558-13-4
257 suppliers
$10.00/1g

603-35-0
733 suppliers
$5.00/5G
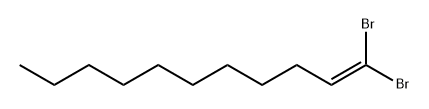
77295-69-3
0 suppliers
inquiry

791-28-6
535 suppliers
$6.00/25g

1034-39-5
162 suppliers
$10.00/1g