
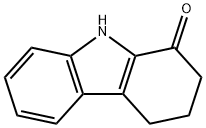
(Z)-2,3,4,9-tetrahydro-1H-carbazol-1-one oxime synthesis
- Product Name:(Z)-2,3,4,9-tetrahydro-1H-carbazol-1-one oxime
- CAS Number:23240-49-5
- Molecular formula:C12H12N2O
- Molecular Weight:200.24
3456-99-3
62 suppliers
$92.00/500mg

23240-49-5
7 suppliers
inquiry
Yield: 97.53%
Reaction Conditions:
Stage #1:1-oxo-2,3,4,9-tetrahydrocarbazole with hydroxylamine hydrochloride;sodium acetate in ethanol;water at 80; for 2 h;Inert atmosphere;
Stage #2:Inert atmosphere;
Steps:
3 Step-I (Z)-2,3,4,9-tetrahydro-1H-carbazol-1-one oxime (4)
To a stirred solution of 2,3, 4, 9-tetrahydro-1H-carbazol-1-one 3 (1.5 g,8.10 mmol) in ethanol (20 mL) added subsequently solution ofhydroxylamine hydrochloride (1.13 g, 16.2 mmol) in water (10 mL)and a solution of sodium acetate (2.19 g, 26.7 mmol) in water(10 mL). After being stirred at 80 °C for 2 h, cooled reaction mixtureto 25-30 °C. Concentrated reaction mixture under reduced pressureand resulting residue was diluted with water (25 mL). Reaction mixture was extracted with ethyl acetate (2x50 mL), combined organic layers were washed with brine (50 mL) dried over anhydrous sulphate and filtered. Filtrate was concentrated under reduced pressure to get title compound as a brown solid (1.58 g, 97.53%).
References:
Kulkarni, Mahesh R.;Mane, Madhav S.;Ghosh, Usha;Sharma, Rajiv;Lad, Nitin P.;Srivastava, Ankita;Kulkarni-Almeida, Asha;Kharkar, Prashant S.;Khedkar, Vijay M.;Pandit, Shivaji S. [European Journal of Medicinal Chemistry,2017,vol. 134,p. 366 - 378]
133-32-4
692 suppliers
$10.00/5g

23240-49-5
7 suppliers
inquiry
