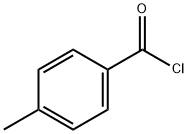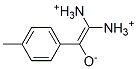
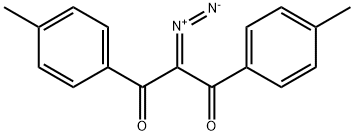
(Z)-2-diazonio-1-(4-methylphenyl)ethenolate synthesis
- Product Name:(Z)-2-diazonio-1-(4-methylphenyl)ethenolate
- CAS Number:17263-64-8
- Molecular formula:C9H8N2O
- Molecular Weight:0
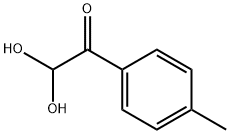
16208-14-3
36 suppliers
$22.00/250mg

17263-64-8
2 suppliers
inquiry
Yield:17263-64-8 96%
Reaction Conditions:
with caesium carbonate;toluene-4-sulfonic acid hydrazide in chloroform at 20; for 0.0833333 h;
Steps:
4.2. General procedure for synthesis of 3 (3a as an example)
General procedure: 4.2. General procedure for synthesis of 3 (3a as an example) A reaction mixture of phenylglyoxal monohydrate 1a (152.2 mg,1.0 mmol), tosylhydrazine 2 (186.2 mg, 1.0 mmol), and Cs2CO3(977.4 mg, 3.0 mmol) in CHCl3 (2 mL) was stirred at room tem-perature for 5 min. Water (50 mL) was then added to the mixture.The organic layer was separated and the aqueous layer wasextracted with EtOAc (50 mL3). The product was dried over an-hydrous Na2SO4 and concentrated under reduced pressure. Theresidue was puried by column chromatography on silica gel (pe-troleum ether/EtOAc5/1) to afford the desired product 3a.
References:
Shu, Wen-Ming;Ma, Jun-Rui;Zheng, Kai-Lu;Sun, Hui-Ying;Wang, Mei;Yang, Yan;Wu, An-Xin [Tetrahedron,2015,vol. 70,# 49,p. 9321 - 9329]
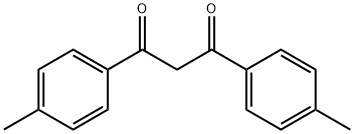
3594-36-3
11 suppliers
$431.00/5g

17263-64-8
2 suppliers
inquiry
105141-03-5
0 suppliers
inquiry

17263-64-8
2 suppliers
inquiry
122-00-9
678 suppliers
$5.00/25g

17263-64-8
2 suppliers
inquiry