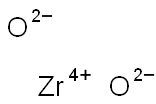
Zirconium dioxide synthesis
- Product Name:Zirconium dioxide
- CAS Number:1314-23-4
- Molecular formula:O2Zr
- Molecular Weight:123.22
Also, the oxide may be prepared in the laboratory by thermal decomposition of zirconium hydroxide or zirconium carbonate:
Zr(OH)4 → ZrO2 + 2H2O
Zr(CO3)2 → ZrO2 + 2CO2
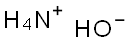
1336-21-6
740 suppliers
$14.56/250ML
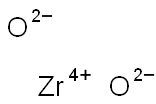
1314-23-4
468 suppliers
$19.50/5g
Yield:-
Steps:
Synthesis of ZrO(OH)2 and ZrO2
Concentrated NH4OH was added to an aqueous solution of ZrOCl2.8H2O (100 gL-1), and the pH of the solution was adjusted to 9 to 10 with vigorous agitation.
The generated emulsion was aged for 24 hours, filtered, and washed thoroughly with water.
The obtained solid precipitate was dried at 100° C. overnight to obtain ZrO(OH)2.
The obtained ZrO(OH)2 was calcined in air at 500° C. for 5 hours to prepare ZrO2.
References:
US2022/62880,2022,A1