ZOXAMIDE synthesis
- Product Name:ZOXAMIDE
- CAS Number:156052-68-5
- Molecular formula:C14H16Cl3NO2
- Molecular Weight:336.64
209809-70-1
0 suppliers
inquiry
87-90-1
468 suppliers
$10.00/10g

156052-68-5
67 suppliers
$135.00/50mg
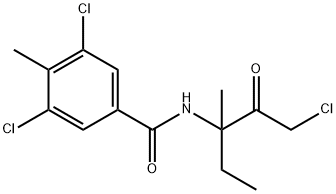
Yield:156052-68-5 87%
Reaction Conditions:
with hydrogenchloride in water;ethyl acetate
Steps:
C.6 Example C6
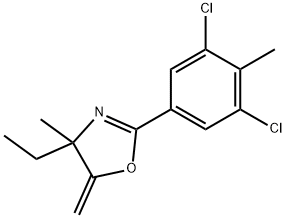
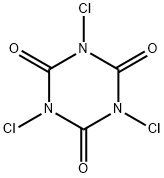
Example C6 Preparation of N-(1-chloro-3-methyl-2-oxopent-3-yl)-3,5-dichloro-4-methylbenzamide A solution of 2-(3,5-dichloro-4-methylphenyl)-4-ethyl-4-methyl-5-methyleneoxazoline (10.0 g, 35.19 mmol) and ethyl acetate (25 mL) was cooled to 50°C using an ice bath. Trichloroisocyanuric acid (2.73 g, 11.73 mmol) was added in several portions over 15 minutes in order to keep the reaction temperature below 40° C. When the addition was complete the reaction mixture was cooled to 20° C, and the ice bath was removed. The reaction was monitored by GC analysis for disappearance of the starting material and was judged to be complete after 1 h. The mixture was filtered; the wetcake was washed with ethyl acetate (5 mL). The filtrate was transferred to a round-bottom flask and heated to 60° C. Hydrochloric acid (0.69 g of a 37% solution) and water (2.2 mL) were added. The reaction mixture was stirred at 60° C for 1.5 h, then at 73° C for an additional 1.5 h. The reaction was then cooled to room temperature. The resulting slurry was stored in a refrigerator overnight. The mixture was filtered, and the solids were rinsed with cold filtrate solution. The filtrate was concentrated to approximately half of its original volume by evaporation under reduced pressure. A seed crystal of product from the first crop was added, and the flask was chilled in a refrigerator at 8° C overnight. The resulting slurry was filtered to obtain a second crop of crystals. Both crops were dried at 60° C under vacuum, yielding N-(1-chloro-3-methyl-2-oxopent-3-yl)-3, 5-dichloro-4-methylbenzamide (10.31 g, 87%) as a white solid, (mp 157-158° C). By following substantially the same procedure, the compounds of Examples C1-C5 and C7-C9 were prepared as shown in Table II.
References:
ROHM AND HAAS COMPANY EP952143, 1999, A1