| Identification | More | [Name]
4,4'-Methylenedianiline | [CAS]
101-77-9 | [Synonyms]
4-(4-AMINOBENZYL)ANILINE
4,4'-DIAMINODIPHENYLMETHANE
4,4'-Diphenylmethanediamine
4,4'-Methylenebisbenzeneamine
4,4'-METHYLENEDIANILINE
4,4-METHYLENEDIANILINE
4,4′-Metylene dianiline
BIS-(4-AMINOPHENYL)METHANE
bis(p-aminophenyl)methane
DADPM
Di-(4-aminophenyl)methane
DIANILINOMETHANE
MDA
methylenebis(aniline)
P,P'-DIAMINODIPHENYLMETHANE
P,P'-METHYLENEDIANILINE
ZERENEX ZX004995
,4’-methylenedianiline
,4'-Methylenebisaniline
4-(4-Aminobenzyl)phenylamine | [EINECS(EC#)]
202-974-4 | [Molecular Formula]
C13H14N2 | [MDL Number]
MFCD00007914 | [Molecular Weight]
198.26 | [MOL File]
101-77-9.mol |
| Chemical Properties | Back Directory | [Appearance]
4,4’-Diaminodiphenylmethane is a pale yel-
low crystalline solid (turns light brown on contact with air)
with a faint amine-like odor. | [Melting point ]
89-91 °C(lit.)
| [Boiling point ]
242 °C2 mm Hg(lit.)
| [density ]
1.15 | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.5014 (estimate) | [Fp ]
430 °F
| [solubility ]
water: soluble | [form ]
neat | [pka]
5.32±0.25(Predicted) | [color ]
White to Light yellow | [Water Solubility ]
Slightly soluble. <0.1 g/100 mL at 19 ºC | [Merck ]
14,2980 | [BRN ]
474706 | [Exposure limits]
ACGIH: TWA 0.1 ppm (Skin)
OSHA: STEL 100 ppb | [LogP]
1.55 at 25℃ | [CAS DataBase Reference]
101-77-9(CAS DataBase Reference) | [IARC]
2B (Vol. 39, Sup 7) 1987 | [NIST Chemistry Reference]
Benzenamine, 4,4'-methylenebis-(101-77-9) | [EPA Substance Registry System]
101-77-9(EPA Substance) |
| Questions And Answer | Back Directory | [Chemical Properties]
4,4'-Methylenedianiline is a light brown crystalline solid with a faint amine odor. lt is very slightly soluble in water and soluble in alcohol, benzene,and ether. lt is combustible when exposed to heat or flame. When heated to decomposition,it emits toxic fumes of aniline and nitrogen oxides (NOx). The dihydrochloride is a crystalline solid that is soluble in water.

4,4'-Methylenedianiline is primarily used to produce 4,4-'methylenedianiline diisocyanate and other polymeric isocyanates which are used to manufacture polyurethane foams.
| [Uses]
4,4'-Methylenedianiline is used in the production of polyurethane foams and epoxy resins. Potential contributor in pulmonary arterial hypertension (PAH) in rats through alteration of the serotenergic transport system. Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants. Dyes and metabolites, Environmental Testing. | [Preparation]
93 g (1 mole) of aniline are dissolved in 96 % alcohol and to the cooled solution with proper stirring 120 g (1 mole) of 40 % formaldehyde solution are gradually added. After the addition is completed a second molecular portion of aniline, 93 g (1 mole) of aniline are added and the reaction is continued until the odor of formaldehyde has disappeared. The complete the reaction the mixture is refluxed for 2 hours. 130 g (1 mole) of aniline hydrochloride are then added, and boiling continued for a further 12 hours. The alcohol is distilled off, the residue made alkaline with the solution of sodium hydroxide and the excess of aniline removed by distillation with superheated steam. The residual oil, which consists of 4,4′-diaminodiphenylmethane, is then purified by solution in dilute hydrochloric acid and reprecipitation with dilute alkali. The 4,4′-diaminodiphenylmethane base is filtered off, washed and dried.
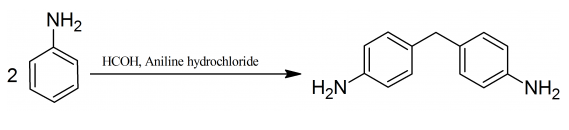
Organic medical chemicals, by M. Barrowliff, 188-189, 1921
|
| Safety Data | Back Directory | [Hazard Codes ]
T,N | [Risk Statements ]
R45:May cause cancer.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R43:May cause sensitization by skin contact.
R48/20/21/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation, and in contact with skin and if swallowed .
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R68:Possible risk of irreversible effects. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 2651 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
BY5425000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29215900 | [Safety Profile]
Confirmed carcinogen
with experimental tumorigenic data. Human
poison by ingestion. Poison by
subcutaneous and intraperitoneal routes.
Human systemic effects by ingestion:
rigidity, jaundice, other liver changes. An eye
irritant. Mutation data reported. It is not
rapidly absorbed through the skin.
Combustible when exposed to heat or
flame. When heated to decomposition it
emits highly toxic fumes of aniline and NOx. | [Hazardous Substances Data]
101-77-9(Hazardous Substances Data) | [Toxicity]
LD50 oral in rabbit: 620mg/kg |
| Hazard Information | Back Directory | [General Description]
A tan flake or lump solid with a faint fishlike odor. May be toxic by inhalation or ingestion, and may be irritating to skin. Insoluble in water. | [Reactivity Profile]
4,4'-DIAMINODIPHENYLMETHANE(101-77-9) polymerizes if heated above 257° F. Incompatible with strong oxidizing agents. 4,4'-DIAMINODIPHENYLMETHANE(101-77-9) is also incompatible with acids. Catalyzes isocyanate-alcohol and epoxide reactions. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. | [Air & Water Reactions]
Oxidizes slowly in air in a reaction catalyzed by light. Somewhat hygroscopic. Insoluble in water. | [Health Hazard]
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | [Potential Exposure]
Used as an intermediate and as a curing
agent. Approximately 99% of the DDM produced is con-
sumed in its crude form (occasionally containing not more
than 50% DDM and ply-DDM) at its production site by reac-
tion with phosgene in the preparation of isocyanates and poly-
isocyanates. These isocyanates and polyisocyanates are
employed in the manufacture of rigid polyurethane foams
which find application as thermal insulation. Polyisocyanates
are also used in the preparation of the semiflexible polyure-
thane foams used for automotive safety cushioning. DDM is
also used as: an epoxy hardening agent; a raw material in the
production of polyurethane elastomers; in the rubber industry
as a curative for Neoprene and as an antifrosting agent (anti-
oxidant) in footwear; a raw material in the production of
Quana nylon; and a raw material in the preparation of poly
(amide-imide) resins (used in magnet wire enamels). | [Fire Hazard]
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ-
ing resuscitation mask) if breathing has stopped and CPR
if heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get
medical attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Shipping]
UN2651 4,40
-Diaminodiphenyl methane, Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
Dust forms and explosive mixture
with air. May polymerize in temperatures .125℃
. A weak
base. Incompatible with strong oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong acids. Flammable gas-
eous hydrogen may be generated in combination with strong
reducing agents, such as hydrides
. | [Description]
Diaminodiphenylmethane is an aromatic diamine used
as a curing agent in epoxy res ins of t11e bisphenol A
type, as in the production of plastics, isocyanates,
adhesives, elastomers, polyuret11ane (elastic and rigid
foams, paints, lacquers, adhesives, binding agents,
synt11etics rubbers, and elastomeric fibers) and butyl
rubber. Diaminodiphenylmet11ane is also a by-product
of azo dyes. It is also possibly formed by hydrolysis of
diphenylmethane-4A'-diisocyanate.
| [Waste Disposal]
Controlled incineration
(oxides of nitrogen are removed from the effluent gas by
scrubbers and/or thermal devices). | [Definition]
ChEBI: An aromatic amine that is diphenylmethane substituted at the 4-position of each benzene ring by an amino group. | [Flammability and Explosibility]
Nonflammable | [Carcinogenicity]
4,4′-Methylenedianiline and its dihydrochloride salt are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals. | [Environmental Fate]
MDA is a pale brown crystalline powder with a faint aminelike
odor. Exposure to air and light results in polymerization and
oxidation of MDA. When heated, MDA produces toxic fumes of
aniline and nitrogen oxides.
Most MDA enters the environment when it is produced or
used to make other compounds. Forty-five percent of the
produced compound is released to deep soil, 52.6% to the air,
and 2.4% to land and water. Once in the environment, it will
be mainly present as tiny particles in the air and it is removed
from the atmosphere by dry deposition, rain, and snow
scavenging. A small amount is transformed by reaction with
hydroxyl radicals. In water, most of MDA will attach to
particles and sediments, and will eventually settle to the
bottom.
The estimated half-life of biodegradation in surface water,
groundwater, and soil is 1–7 days, 2–14 days, and 1–7 days,
respectively. With respect to aquatic ecosystems, bioconcentration
factor values of 3.0–14 suggest that bioconcentration
is low, in addition this compound does not tend
to build up in the food chain. When released to soil, it is
expected to have slight to no mobility. Similarly, volatilization
from both moist and dry soil surfaces is not expected to be
important. | [storage]
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from strong oxidizers (such as chlorine, bromine, and fluorine). A regulated, marked areashould be established where this chemical is handled, used,or stored in compliance with OSHA Standard 1910.1045. | [Purification Methods]
Crystallise the amine from water, 95% EtOH or *benzene. [Beilstein 13 IV 390.] | [Toxicity evaluation]
Currently, the mechanism of action is not completely understood
and has been mostly assessed from information on
structurally similar compounds. Many of the toxic properties of
MDA have been attributed to metabolic intermediates, as these
compounds are metabolically activated by N-oxidation to
metabolites, such as N-hydroxymethylenedianiline, that react
with DNA, RNA, and proteins.
Different studies suggest that both liver and thyroid are
targets for MDA toxicity in humans and animals. Liver
toxicity has been linked to impair mitochondrial function
and structure, apoptosis, and increased oxidative stress. It
may be caused by a reactive electrophile formed during
metabolism since liver has the enzymatic routes necessary for
such activation. Experimental studies indicate that biliary
epithelial cells are damaged earlier than parenchymal cells
and bile is a major route of exposure to MDA. The mechanism
of thyroid toxicity has not yet been resolved. It has
been observed that MDA exposure can induce a slight
decrease in thyroid hormones in rats, thus triggering secretion
of thyroid-stimulating hormone (TSH), which induced
thyroid hyperplasia. Some of the induced adverse effects
observed after MDA exposure (e.g., reduced food consumption,
lower body weight gain, and effects on red cells,
lymphocytes, and clotting parameters) could be explained as
secondary responses.
MDA is carcinogenic to animals. The mechanism of liver or
thyroid tumor development remains unclear. Even if cell
injury may give indications of a nongenotoxic mechanism, it
remains still unproved, and there are also positive genotoxic
data in vitro and in vivo which indicate that a genotoxic
mechanism may be related to the formation of a reactive
metabolic intermediate cannot be excluded. Regarding thyroid
cancer, hypersecretion of TSH may have a contribution to
tumor formation. | [Toxics Screening Level]
The initial risk screening level (IRSL) for 4,4’-methylenedianiline (CAS # 101-77-9) is 0.022
μg/m3 annual averaging time and the secondary risk screening level (SRSL) is 0.22 μg/m3
annual averaging time. |
|
|