| Identification | More | [Name]
Rhein | [CAS]
478-43-3 | [Synonyms]
1,8-dihydroxy-3-carboxyanthraquinone
1,8-DIHYDROXY-9,10-ANTHRAQUINONE-3-CARBOXYLIC ACID
1,8-DIHYDROXYANTHRAQUINONE-3-CARBOXYLIC ACID
4,5-DIHYDROXYANTHRAQUINONE-2-CARBOXYLIC ACID
9,10-DIHYDRO-4,5-DIHYDROXY-9,10-DIOXO-2-ANTHRACENECARBOXYLIC ACID
CASSIC ACID
RHEIN
RHUBARB EXTRACT
RHUBARB YELLOW
TIMTEC-BB SBB001152
9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthracenecarboxylicaci
9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroicaci
chrysazin-3-carboxylicacid
monorhein
rheicacid
9,10-dihydro-4,5-dihydroxy-9,10-dioxoanthracene-2-carboxylic acid
RHEIN, TECH.
4,5-Dihydroxy-2-anthraquinonecarboxylic Acid
NSC 38629
RHEIN90%,95% | [EINECS(EC#)]
207-521-4 | [Molecular Formula]
C15H8O6 | [MDL Number]
MFCD00009618 | [Molecular Weight]
284.22 | [MOL File]
478-43-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Yellowish Brown Solid | [Melting point ]
≥300 °C (lit.) | [Boiling point ]
346.72°C (rough estimate) | [density ]
1.3269 (rough estimate) | [refractive index ]
1.4413 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
neat | [pka]
3.17±0.20(Predicted) | [color ]
Yellow to Dark Orange | [Water Solubility ]
<0.1 g/100 mL at 17 ºC | [Usage]
Found in the free state and as glucoside in Rheum spp, Polygonaceae (rhubarb) and in Senna leaves.
A potential antioxidant resource: endophytic fungi from medicinal plants. | [λmax]
432nm(MeOH)(lit.) | [Merck ]
13,8260 | [BRN ]
2222155 | [Stability:]
Hygroscopic | [InChIKey]
FCDLCPWAQCPTKC-UHFFFAOYSA-N | [LogP]
4.290 (est) | [CAS DataBase Reference]
478-43-3(CAS DataBase Reference) | [EPA Substance Registry System]
478-43-3(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
2
| [RTECS ]
CA9516000
| [HS Code ]
29189900 | [Safety Profile]
A poison by intravenous route. Low toxicity by ingestion. When heated to decomposition it emits acrid smoke and irritating vapors. |
| Hazard Information | Back Directory | [General Description]
Yellow needles (from methanol) or yellow-brown powder. | [Reactivity Profile]
RHEIN(478-43-3) forms a red potassium salt and a pink sodium salt. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Low toxicity by ingestion.
| [Fire Hazard]
Flash point data for this chemical are not available; however, RHEIN is probably combustible. | [Chemical Properties]
Yellowish Brown Solid | [Definition]
ChEBI: Rhein is a dihydroxyanthraquinone. | [Biochem/physiol Actions]
Constituent that is enriched in rhubarb with anti-inflammatory, anti-osteoarthritic, and anti-cancer activity. It reduces IL-1β production and secretion, caspase-3 activity, inducible nitric oxide synthase activity, and phosphorylation of c-Jun and c-Jun NH2-terminal kinase (JNK). | [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Physical and Chemical Properties]
The chemical name of Rhein is 1,8-dihydroxy anthraquinone-3-carboxylic acid, with the molecular formula C15H8O6 and the molecular weight of 284.21. It becomes yellow acicular crystal after sublimation, with the melting point 321~322 ℃ and decomposition temperature 330 ℃ and UVλmax (methanol) 229, 258, 435nm. It is soluble in alkali and pyridine, and slightly soluble in alcohol, ether, benzene, chloroform, petroleum ether, and insoluble in water. It can form red sylvine and pink sodium salt, and form a red precipitate with calcium hydroxide and barium hydroxide.
Production Method: being acquired from the hydrolysis of Rhein diacetic ester.
diethyl acetate. Uses: Current clinical treatment of rheumatic drugs often contain Rhein.
Rhein is extracted from the root of the plant Rheum palmatum L. in Polygonaceae family, which is a anthraquinones and has the functions of antibacterial, anti-cancer, cathartic, and diuretic. The contents of rhein, aloe-emodin and rheum emodin in rhubarb are listed in the following table:
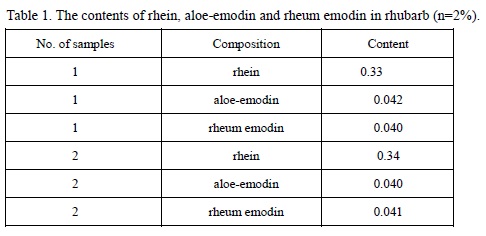
Table 1. The contents of rhein, aloe-emodin and rheum emodin in rhubarb (n=2%). | [Pharmacological effects]
There are chemical compounds chrysophanic acid, rhein, aloe-emodin, rheum emodin, aloe emodin, and Sennoside in rhubarb.
Both the rhein and rheum emodin have the anti-tumor effect, especially a strong inhibitory effect for melanoma and they have certain inhibition on breast cancer and ehrlich’s ascites carcinoma. When rhein was applied to intratumoral administration in mice with breast cancer, there was a significant damaging in the cancer tissue. The inhibition rate of 5mg/kg rhein and rheum emodin on murine melanoma was 76% and 73%, respectively. Rheum emodin has significant competitive inhibition on tyrosinase, and this inhibition may be one of the mechanisms why rhubarb has the anti-melanoma effect. At the concentration of 10μg/ml, Rheum emodin significantly inhibited the cell division and DNA biosynthesis of human lung cancer A-549 cells. After the subcutaneous injection of crude extracts of rhubarb, an inhibition of mouse sarcoma S37 was found. The inhibition rate of Rhein on ehrlich’s ascites carcinoma and sarcoma S180 in mice was 15% and 21%, respectively. The inhibition rate of hot water extracts of Rhubarb on sarcoma S180 in mice was 48.8%.
Rhein has inhibition effect on mouse leukemia P388. Rhein, aloe-emodin and rheum emodin are extracted from rhubarb and these three anthraquinone derivatives could minimize the amount of ascites and the number of cancer cells in different extent in mice with tumors, among which the effect of rhein was most obvious and the effect of aloe-emodin was poorer, with a almost parallel relationship with prolonged survival time. The inhibition of rhein and rheum emodin on biosynthesis of DNA, RNA and protein was stronger, whereas the inhibition of aloe-emodin was weaker. | [Uses]
Medical intermediate;raw materials of health food.
|
|
|