| Identification | More | [Name]
Acetazolamide | [CAS]
59-66-5 | [Synonyms]
2-acetamido-5-sulfamoyl-1,3,4-thiadiazole
2-ACETAMINO-1,3,4-THIADIAZOLE-5-SULFONAMIDE
2-ACETYLAMINO-1,3,4-THIADIAZOLE-5-SULFONAMIDE
5-ACETAMIDO-1,3,4-THIADIAZOLE-2-SULFONAMIDE
ACETAMIDE N-(5-(AMINO SULPHONYL)-1,3,4 THIADIAZOL-2-YL)
ACETAZOLAMIDE
DIACARB
LABOTEST-BB LT00012571
N-(5-[AMINOSULFONYL]-1,3,4-THIADIAZOL-2-YL)ACETAMIDE
N-[5-(AMINOSULFONYL)-1,3,4-THIADIOZOL-2-YL]-ACETAMIDE
N-(5-SULFAMOYL-1,3,4-THIADIAZOL-2-YL)ACETAMIDE
N-(5-SULFAMOYL-1,3,4-THIADIAZOL-2-YL)ETHANAMIDE
1,3,4-Thiadiazole-2-sulfonamide, 5-acetamido-
2-Acetamido-5-sulfonamido-1,3,4-thiadiazole
4-Diamox
4-thiadiazole-2-sulfonamide,5-acetamido-3
5-Acetamide-1,3,4-thiadiazole-2-sulfonamide
5-Acetamido-1,3,4-thiadiazol-2-sulfonamide
6063
Acetamide, N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)- | [EINECS(EC#)]
200-440-5 | [Molecular Formula]
C4H6N4O3S2 | [MDL Number]
MFCD00003105 | [Molecular Weight]
222.25 | [MOL File]
59-66-5.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/38:Irritating to eyes and skin .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
2811 | [WGK Germany ]
2 | [RTECS ]
AC8225000 | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29350090 | [Safety Profile]
Poison by subcutaneous and intravenous routes. Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: dyspnea. An experimental teratogen by many routes. Other experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx, and SOx,. A carbonic anhydrase inhibitor and dmretic used to treat glaucoma. | [Hazardous Substances Data]
59-66-5(Hazardous Substances Data) | [Toxicity]
LD50 oral in mouse: 4300mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Acetyl chloride-->Sulfuryl chloride-->Sodium metabisulfite-->Ammonium thiocyanate-->2-Acetylamino-5-mercapto-1,3,4-thiadiazole-->Butanedioic acid,1-[1-[[(1-methylethyl)amino]methyl]-2-(1-naphthalenyloxy)ethyl] ester-->Benzoic acid, 2-[[[[5-(acetylamino)-1,3,4-thiadiazol-2-yl]sulfonyl]amino]carbonyl]-, 1-[[(1-methylethyl)amino]methyl]-2-(1-naphthalenyloxy)ethyl ester-->5-AMINO-1,3,4-THIADIAZOLE-2-SULFONAMIDE-->2-ACETAMIDO-5-BENZYLTHIO-1 3 4--->2-(ACETAMIDO)-5-(CHLOROSULFONYL)-1,3,4-THIADIAZOLE |
| Hazard Information | Back Directory | [General Description]
White to yellowish-white fine crystalline powder. No odor or taste. | [Reactivity Profile]
A weak acid and a diazo derivative. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, ACETAZOLAMIDE is probably combustible. | [Chemical Properties]
White Solid | [Originator]
Diamox ,Lederle,US ,1953 | [Uses]
Acetazolamide acts as a carbonic anhydrase inhibitor which increases cerebral blood flow. It inhibits water permeability of membranes by interacting with aquaporins. It is used for the medical treatment of glaucoma, epileptic seizure, idiopathic intracranial hypertension, altitude sickness, cystinuria. | [Uses]
Acetazolamide is used for epilepsy in the absence of attacks and also in conjunction with
other antiepileptic drugs. | [Uses]
carbonic anhydrase inhibitor, diuretic, antiglaucoma | [Definition]
ChEBI:Acetazolamide is a sulfonamide, a member of thiadiazoles and a monocarboxylic acid amide. It has a role as a diuretic, an anticonvulsant and an EC 4.2.1.1 (carbonic anhydrase) inhibitor. It is a conjugate acid of an acetazolamide(1-). It derives from a hydride of a 1,3,4-thiadiazole. | [Manufacturing Process]
According to REM, hydrazine hydrate is reacted with 2 mols of ammonium
thiocyanate to produce 1,2-bis(thiocarbamoyl)hydrazine which by loss of
ammonia and rearrangement produces 5-amino-2-mercapto-1,3,4-thiadiazole.
That compound is acetyled with acetic anhydride.
Then, as described in US Patent 2,554,816, the 2-acetylamido-5-mercapto-
1,3,4-thiadiazole is converted to the sulfonyl chloride by passing chlorine gas
into a cooled (5-10°C) solution in 33% acetic acid (66 parts to 4 parts of
mercapto compound) used as a reaction medium. Chlorine treatment is
continued for two hours. The crude product can be dried and purified by
recrystallization from ethylene chloride. The pure compound is a white
crystalline solid, MP 194°C, with decomposition, when heated rapidly. The
crude damp sulfonyl chloride is converted to the sulfonamide by addition to a
large excess of liquid ammonia. The product is purified by recrystallization
from water. The pure compound is a white, crystalline solid, MP 259°C, with
decomposition. The yield of sulfonamide was 85% of theory based on
mercapto compound.
An alternative process is described in US Patent 2,980,679 as follows. 15
grams of finely powdered 2-acetylamino-1,3,4-thiadiazole-5-mercaptain are
suspended in 200 ml of water containing 4 grams of potassium bromide. From
0.5 to 1 gram of ferric chloride are subsequently added. The mass is
energetically stirred and 52 grams of liquid bromide are added by increments
for about 45 minutes, while keeping the reaction temperature below 10°C,
and, preferably, at 4-8°C by employing a cooling bath. Stirring is continued
for a further 10 minutes, then the 2-acetylamino-1,3,4-thiadiazole-5-
sulfobromide is collected on a funnel equipped with a porous diaphragm,
thoroughly washed with cold water and finally subjected to amidation with
liquid ammonia. The reaction mixture is allowed to stand for a certain period,
then the ammonia is evaporated, after which the residue is taken up with
diluted ammonia and, after decolorizing with carbon, the sulfonamide is
precipitated with hydrochloric acid. The yield of crude sulfonamide obtained
with this process, with respect to the starting mercapto compound is abut
84%. If the amidation is carried out with 33% aqueous ammonia, the yield is
slightly lower. | [Brand name]
Diamox (Duramed). | [Therapeutic Function]
Carbonic anhydrase inhibitor, Diuretic, Antiglaucoma | [Biochem/physiol Actions]
Inhibits water permeability of membranes by interacting with aquaporins | [Mechanism of action]
Acetazolamide is an aromatic sulfonamide used as a carbonic anhydrase inhibitor. It facilitates
production of alkaline urine with an elevated biocarbonate, sodium, and potassium ion concentrations.
By inhibiting carbonic anhydrase, the drug suppresses reabsorption of sodium ions
in exchange for hydrogen ions, increases reflux of bicarbonate and sodium ions and reduces
reflux of chloride ions. During this process, chloride ions are kept in the kidneys to cover of
insufficiency of bicarbonate ions, and for keeping an ion balance. Electrolytic contents of fluid
secreted by the kidneys in patients taking carbonic anhydrase inhibitors are characterized by
elevated levels of sodium, potassium, and bicarbonate ions and a moderate increase in water
level. Urine becomes basic, and the concentration of bicarbonate in the plasma is reduced. | [Clinical Use]
Acetazolamide was the first of the carbonic anhydrase inhibitors to be introduced as an orally effective diuretic, with a diuretic effect that lasts approximately 8 to 12
hours. As mentioned earlier, its diuretic action is limited because of the systemic acidosis it produces. Acetazolamide
reduces the rate of aqueous humor formation and is used primarily for reducing intraocular pressure in the treatment of glaucoma. The dose is 250 mg to 1 g per day. | [Synthesis]
Acetazolamide is 5-acetamido-1,3,4-thiadiazole-2-sulfonamide (9.7.5).
The synthesis of acetazolamide is based on the production of 2-amino-5-mercapto-1,3,
4-thiadiazole (9.7.2), which is synthesized by the reaction of ammonium thiocyanate and
hydrazine, forming hydrazino-N,N�-bis-(thiourea) (9.7.1), which cycles into
thiazole (9.7.2) upon reaction with phosgene. Acylation of (9.7.2) with acetic anhydride
gives 2-acetylamino-5-mercapto-1,3,4-thiadiazol (9.7.3). The obtained product is
chlorinated to give 2-acetylamino-5-mercapto-1,3,4-thiadiazol-5-sulfonylchloride
(9.7.4), which is transformed into acetazolamide upon reaction with ammonia (9.7.5)
[24,25].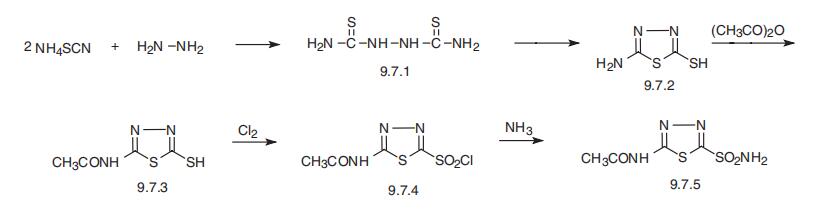
| [Veterinary Drugs and Treatments]
Acetazolamide has been used principally in veterinary medicine
for its effects on aqueous
humor production in the treatment of
glaucoma, metabolic alkalosis, and for its diuretic action. It may
be useful as an adjunctive treatment for syringomyelia in dogs.
Acetazolamide’s use in small animals is complicated by a relatively
high occurrence of adverse effects.
In horses, acetazolamide is used as an adjunctive treatment for
hyperkalemic periodic paralysis (HYPP).
In humans, the drug has been used as adjunctive therapy for epilepsy
and for acute high-altitude sickness. | [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: high dose aspirin reduces excretion (risk
of toxicity).
Anti-arrhythmics: increased toxicity if hypokalaemia
occurs.
Antibacterials: effects of methenamine antagonised.
Antiepileptics: increased risk of osteomalacia
with phenytoin and phenobarbital; concentration
of carbamazepine and possibly fosphenytoin and
phenytoin increased.
Antihypertensives: enhanced hypotensive effect.
Antipsychotics: increased risk of ventricular
arrhythmias due to hypokalaemia.
Atomoxetine: increased risk of ventricular
arrhythmias due to hypokalaemia.
Beta-blockers: increased risk of ventricular
arrhythmias due to hypokalaemia with sotalol.
Cardiac glycosides: increased toxicity if hypokalaemia
occurs.
Ciclosporin: possibly increases ciclosporin
concentration.
Cytotoxics: alkaline urine increases methotrexate
excretion; increased risk of ventricular arrhythmias
due to hypokalaemia with arsenic trioxide; increased
risk of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium: lithium excretion increased. | [Metabolism]
Acetazolamide is tightly bound to carbonic anhydrase
and accumulates in tissues containing this enzyme,
particularly red blood cells and the renal cortex. It is also
bound to plasma proteins. It is excreted unchanged in the
urine, renal clearance being enhanced in alkaline urine. | [storage]
Store at -20°C | [Purification Methods]
It is recrystallised from water. [Roblin & Clapp J Am Chem Soc 72 4890 1950, Beilstein 27 III/IV 8219.] |
| Questions And Answer | Back Directory | [Description]
Acetazolamide is a drug used for the treatment of glaucoma,epilepsy,altitude sickness,periodic paralysis, chronic macular edema, idiopathic intracranial hypertension, andheart failure. It can also been used for the treatment of altitude sickness, increased intracranial pressure and neuromuscular disorders. In addition, it also has significant effect of diuretic. It belongs to the carbonic anhydrase inhibitorfamilies of medication. It works by decreasing the amount ofhydrogen ionsandbicarbonatein the body.
| [References]
Forwand, S. A., et al. "Effect of acetazolamide on acute mountain sickness." New England Journal of Medicine279.16(1968):839.
Cox, S. N., E. Hay, and A. C. Bird. "Treatment of chronic macular edema with acetazolamide." Archives of Ophthalmology 106.9(1988):1190.
Supuran, Claudiu T. "Acetazolamide for the treatment of idiopathic intracranial hypertension." Expert Review of Neurotherapeutics15.8(2015):851.
Kassamali, R, and D. A. Sica. "Acetazolamide: a forgotten diuretic agent." Cardiology in Review 19.6(2011):276.
Lucas, M., and M. Brown. "Acetazolamide Reduces Hospital Admissions and Length of Stay in Refractory Heart Failure Patients." Heart Lung & Circulation 20.Suppl 2(2011):S6-S6.
https://www.rxlist.com/acetazolamide-drug.htm
https://en.wikipedia.org/wiki/Acetazolamide
|
|
|