| Identification | More | [Name]
Cesium chloride | [CAS]
7647-17-8 | [Synonyms]
CAESIUM CHLORIDE
CESIUM CHLORIDE
CESIUM CHLORIDE IN 0.1 N HCL
CSCL
Cesium chloride (CsCl)
Cesium monochloride
cesiumchloride(cscl)
cesiummonochloride
Dicesium dichloride
dicesiumdichloride
Tricesium trichloride
tricesiumtrichloride
Caesiumchlorid
Caesium chloride 99+ %
Cesium Chloride, MB Grade (1.01548)
Cesium chloride, Molecular Biology Grade
Cesium Chloride, ULTROL Grade
Cesium chloride,(99.9% Cs)
Cesium ion chromatography standard solution Fluka
Cesiumchlorid | [EINECS(EC#)]
231-600-2 | [Molecular Formula]
ClCs | [MDL Number]
MFCD00010955 | [Molecular Weight]
168.36 | [MOL File]
7647-17-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White/clear cryst. powder | [Melting point ]
645 °C (lit.) | [Boiling point ]
1290 °C
| [density ]
3.983
| [vapor density ]
5.8 (vs air)
| [refractive index ]
1.6418 | [Fp ]
1303°C | [storage temp. ]
2-8°C
| [solubility ]
H2O: 3 M at 20 °C, clear, colorless
| [form ]
beads
| [color ]
White | [Specific Gravity]
3.988 | [Odor]
Odorless | [PH]
5.0-7.5 (25℃, 3M in H2O) | [PH Range]
6.0 - 7.5 | [Stability:]
Stable. Deliquescent. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. | [Water Solubility ]
1860 g/L (20 ºC) | [Sensitive ]
Hygroscopic | [Usage]
For molecular genetic applications used in preparing nucleic acids for subsequent hybridization or cloning experiments | [λmax]
λ: 260 nm Amax: 0.02
λ: 280 nm Amax: 0.02 | [Merck ]
14,2011 | [InChIKey]
AIYUHDOJVYHVIT-UHFFFAOYSA-M | [CAS DataBase Reference]
7647-17-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Cesium chloride(7647-17-8) | [EPA Substance Registry System]
7647-17-8(EPA Substance) |
| Questions And Answer | Back Directory | [Description]
Cesium chloride is the inorganic compound with the formula CsCl. This colorless solid is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions. Cesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Cesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
Cesium Chloride is a chemical reagent used in nuclear and radiologic medical treatments (e.g. Cancer radiation therapy, high pH therapy), in the diagnosis of myocardial infarction, and as a solute for ultracentrifugation.
Cesium chloride is widely used medicine structure in isopycnic centrifugation for separating various types of DNA. It is a reagent in analytical chemistry, where it is used to identify ions by the color and morphology of the precipitate. | [Physical properties]
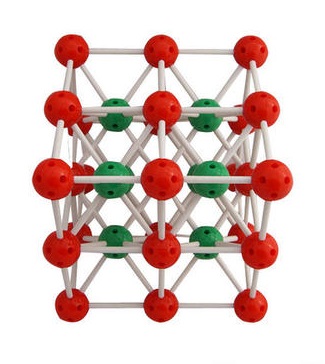
White cubic crystal; hygroscopic; density 3.99 g/cm3; melts at 645°C; vaporizes at 1297°C; very soluble in water, soluble in ethanol. | [Crystal structure]
The Cesium chloride structure is composed of interlocking simple cubic lattices of anions and cations. It is the case that in a cubic 1:1 solid where one atom type is much larger than the other that the Cesium chloride type lattice is obtained, it can be thought of as a combination of basketballs and golf balls packed in a cubic manner with the golf balls in the gaps between the basketballs. If the two atom types are similar in size (imagine field hockey balls packed with tennis balls) then in the cubic lattice the structure will be like that of sodium chloride. | [Uses]
Cesium chloride is used for the preparation of electrically conducting glasses. It is also used to make solutions for the separation of RNA from DNA by density gradient centrifugation.
Cesium chloride is also widely used in the centrifugation of DNA, in a technique known as isopycnic centrifugation. In this method, a Cesium chloride solution is centrifuged, allowing centrifugal and diffusive forces to establish a concentration gradient (and thus a density gradient) within the centrifuge tube. When DNA is centrifuged in this solution, fragments of DNA will migrate down the tube until they reach a zone where the density of the DNA is equal to the density of the solution. At this point, the DNA will stop migrating. This allows separation of DNA of different densities (e.g. DNA fragments with differing A-T or G-C content). Cesium chloride (non-radioactive) is also promoted as an alternative cancer therapy. These claims are not supported by scientific evidence.
Cesium chloride (CsCl) is a mineral salt that is sometimes taken either by mouth, or by injection into the body, by cancer patients who seek alternative treatments. However, no CsCl products have been approved by FDA to treat cancer or other diseases. | [Preparation]
Cesium chloride can be prepared by the reaction of caesium hydroxide or caesium carbonate with hydrochloric acid: the resulting salt is purified by recrystallization.
| [Mechanism of Action]
Proponents of cesium chloride therapy claim that it exerts antitumor effects by increasing the intracellular pH of tumor cells. The resulting alkaline environment is thought to prevent cancer cells from undergoing mitosis eventually resulting in cell death. Cesium causes hypokalemia by inhibiting potassium channels used for absorption of dietary potassium and for re-absorption of renal potassium. Cesium may also cause hypokalemia indirectly via loss of potassium due to repetitive diarrhea. Intravenous administration of cesium has been shown to cause arrhythmias in animal models.
|
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R68:Possible risk of irreversible effects.
R36/38:Irritating to eyes and skin . | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
2923 | [WGK Germany ]
2
| [RTECS ]
FK9625000
| [F ]
3 | [TSCA ]
Yes | [HS Code ]
28273980 | [Safety Profile]
Moderately toxic by
ingestion and intraperitoneal routes.
Experimental reproductive effects. Mutation
data reported. Reacts violently with BF3. See
also CESIUM. When heated to
decomposition it emits toxic fumes of Cl-. | [Hazardous Substances Data]
7647-17-8(Hazardous Substances Data) | [Toxicity]
LD50 i.p. in rats: 1.5 g/kg (Cochran) |
| Hazard Information | Back Directory | [Chemical Properties]
White/clear cryst. powder | [History]
Someone has found that CsCl is described as showing a blue glow in the dark. It is interpreted that light can be caused by the collision of subatomic particles that escape from the nucleus of cesium 137 and collide with electrons located on the periphery of the atom, so the energy of the electrons increases, to return to the previous equilibrium state, the electrons release the excess of energy emitting light.
It is also explained that because it is a highly active light source used in cancer treatment, blue light can be seen by the naked eye. The blue light is due to fluorescent UV dominant optical emission caused by beta, gamma, and Ba X-ray emissions from within the same excited 137-Cs atoms by a previously unknown atomic phenomenon, now known as Padmanabha Rao effect. But looking at Wikipedia, the IAEA report does not explain whether the blue light emitted by 137-c is fluorescence or Cerenkov radiation.
| [Application]
Cesium chloride has been used in the generation of discontinuous gradient for the purification of Cryptosporidium oocysts and as a component of acetamide medium for fungal selection.
Cesium chloride may be employed in gel-electrophoresis and RNA purification studies. It may be employed for the isolation of bacterial plasmid from Agrobacterium spp. | [Definition]
ChEBI: The inorganic chloride salt of caesium; each caesium ion is coordinated by eight chlorine ions. | [Brand name]
Cescan-131 (Abbott). | [General Description]
Cesium chloride is a cesium halide. Cesium halides can be prepared by reacting cesium carbonate with corresponding hydrohalic acids. Cesium chloride ultracentrifugation method has been reported for the extraction of RNA from cellular fractions. | [Health Hazard]
Whereas conventional caesium chloride has a rather low toxicity to humans and animals, the radioactive form easily contaminates the environment due to the high solubility of CsCl in water. Spread of 137CsCl powder from a 93-gram container in 1987 in Goiania, Brazil, resulted in one of the worst-ever radiation spill accidents killing four and directly affecting 249 people. | [Flammability and Explosibility]
Nonflammable | [Biochem/physiol Actions]
Oral intake of cesium chloride is known to increase the pH in cancer cells. Mild toxicity of cesium chloride might cause hypotension, gastrointestinal distress, numbness and syncope. It also leads to severe hypokalemia, hypomagnesemia, acute heart attack and polymorphic ventricular tachycardia in episodes. | [Purification Methods]
It is soluble in H2O but can be purified by crystallisation from H2O [solublity in g percent: 162.3(0.7o), 182.2(16.2o) and 290(at bp 119.4o)] and dried in high a vacuum. It is soluble in EtOH and is deliquescent; keep it in a tightly closed container. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 951-955 1963.] For further purification of CsCl, a concentrated aqueous solution of the practically pure reagent is treated with an equivalent weight of I2 and Cl2 is bubbled into the solution until preciptation of CsCl2I is complete. Recrystallisation yields a salt which is free from other alkali metals. It is then decomposed to pure CsCl on heating. [Harned & Schupp J Am Chem Soc 52 3886 1930.] It can also be recrystallised from acetone/water, or from water (0.5mL/g) by cooling in a CaCl2/ice bath. Dry it at 78o under vacuum. |
|
|