| Identification | Back Directory | [Name]
Pentaerythritol tetranitrate | [CAS]
78-11-5 | [Synonyms]
PETN
CHOT
Extex
Nexit
elpetn
Erinit
Antora
SDM 23
SDM 35
Vasolat
xtx8003
sdmno23
sdmno35
Terpate
vasitol
vaso-80
Pentran
Peridex
Perityl
Angicap
Angitet
Lentrat
Niperyl
Niperyt
Nexol-E
Omnitox
Pencard
Ovadziak
Niperyth
nitrinol
Kaytrate
Metranil
mikardol
Mycardol
Duotrate
PENTRITE
Pentrate
pentriol
Pentafin
pentarit
Penthrit
sdmno.23
sdmno.35
Subicard
Tanipent
XTX 8003
Tetrasule
Peritrate
Penthrite
Pen-Tetra
Pentritol
Pentryate
Pentrinat
Prevangor
Quintrate
Rythritol
pentral80
Pergitral
Arcotrate
Baritrate
Cardiacap
Hasethrol
Lowetrate
Nitropent
nci-c55743
Dipentrate
NITROPENTA
Peridex-LA
Pentitrate
Pentral 80
Pentafilin
Vasodiatol
Tentrate-20
Nitrotalans
Pentanitrol
pentryate80
Deltrate-20
Dilcoran-80
Neo-Corovas
Martrate-45
Nexit-stark
Nicochloran
Nitropenton
Myotrate 10
myotrate"10"
Pentanitrine
Pentestan-80
tranited-lay
Tranite D-Lay
Nitropenta 7W
Nitro-Riletten
nitropenthrite
Nitropenta, PETN
vaso-80unicelies
Vaso-80 Unicelles
Pentritol tempules
pentetrateunicelles
Nitropentaerythrite
Nitropentaerythritol
Pentetrate Unicelles
Pentaerythritol tetran
component of Sdm no. 35
neopentanetetraylnitrate
Tetranitropentaerythrite
Tetranitropentaerythritol
Neopentanetetrayl nitrate
Pentaerythrite tetranitrate
pentaerithrityltetranitrate
PENTAERYTHRITOL TETRANITRATE
PENTAERYTHRITYL TETRANITRATE
Pentaerithrityl Tetranitrate
component of Miltrate, sdm no. 23
pentaerythritoltetranitrate,diluted
Pentaerythritol tetranitrate mixture
pentaerythritol tetranitrate P.E.T.N.
Nitropenta (Pentaerythritol tetranitrate)
1,3-dinitrato-2,2-bis(nitratomethyl)propane
2,2-Bishydroxy-methyl-1,3-propanedioltetranitrate
2,2-bisdihydroxymethyl-1,3-propanedioltetranitrate
2,2-bis(hydroxymethyl)-1,3-propanedioltetranitrate
[3-nitrooxy-2,2-bis(nitrooxymethyl)propyl] nitrate
2,2-Bis[(nitrooxy)methyl]-1,3-propanedioldini-trate
2,2-Bis(Hydroxymethyl)-1,3-propanediol tetranitrate
2,2-Bis(hydroxy-methyl)-1,3-propanedioltetranitrate
1,3-Propanediol, 2,2-bis[(nitrooxy)methyl]-, dinitrate
1-3 Propanediol,2,2-bis(nitroxy)methyl-,dinitrate(ester)
3-propanediol,2,2-bis((nitrooxy)methyl)-dinitrate(ester)
3-propanediol,2,2-bis[(nitrooxy)methyl]-dinitrate(ester)
2,2-bis((nitrooxy)methyl)-1,3-propanedioldinitrate(ester)
2,2-Bis[(nitrooxy)methyl]-1,3-propanediol dinitrate (ester)
nitric acid [3-nitrooxy-2,2-bis(nitrooxymethyl)propyl] ester
1,3-Propanediol, 2,2-bis[(nitrooxy)methyl]-, dinitrate (ester)
pentaerythritetetranitrate,[wetwith>=25%waterordesensitizedwith>=15%phlegmatizer] | [EINECS(EC#)]
201-084-3 | [Molecular Formula]
C5H8N4O12 | [MDL Number]
MFCD00058681 | [MOL File]
78-11-5.mol | [Molecular Weight]
316.14 |
| Chemical Properties | Back Directory | [Appearance]
Pentaerythrite tetranitrate (PETN) is a high
explosive, especially when dry. PETN is a sand-like, white
crystalline solid. Piratically odorless. | [Melting point ]
140° | [Boiling point ]
455.59°C (rough estimate) | [density ]
d420 1.773 | [refractive index ]
1.7900 (estimate) | [storage temp. ]
?20°C | [solubility ]
Practically insoluble in water, soluble in acetone, slightly soluble in ethanol (96 per cent). The solubility of diluted pentaerythrityl tetranitrate depends on the diluent and its concentration. | [color ]
Crystals or prisms from Me2CO/EtOH | [Stability:]
Stable, but decomposes readily and possibly explosively if heated. Incompatible with strong oxidizing agents. Store cold and keep away from sources of ignition. | [Water Solubility ]
2mg/L(temperature not stated) | [NIST Chemistry Reference]
Pentaerythritol, tetranitrate(78-11-5) | [EPA Substance Registry System]
1,3-Propanediol, 2,2-bis[(nitrooxy)methyl]-, dinitrate (ester)(78-11-5) |
| Hazard Information | Back Directory | [Chemical Properties]
white crystalline powder | [Definition]
ChEBI: A pentaerythritol nitrate in which all four hydroxy groups of pentaerythritol have been converted to the corresponding nitrate ester. It is a vasodilator with properties similar to those of glyceryl trinitrate, but with a more prolonged duration of action,
and is used for treatment of angina pectoris. It is also one of the most powerful high explosives known and is a component of the plastic explosive known as Semtex. | [Uses]
Mainly in the manufacture of detonating fuse (Primacord), a waterproof textile filled with powdered PETN. | [Reactivity Profile]
Pure PENTAERYTHRITE TETRANITRATE is an explosive. Severe explosion hazard when shocked or exposed to heat. Explodes when heated to 205-215°C. Highly dangerous when mixed with oxidizing agents. On decomposition Pentaerythritol tetranitrate emits highly toxic fumes of NOx (Sax and Lewis, 1987 pp. 699-700). | [Health Hazard]
Some are toxic and may be fatal if inhaled, swallowed or absorbed through skin. Contact may cause burns to skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | [Potential Exposure]
First introduced following WWII,
PETN shares the same chemical family as nitroglycerine.
It is 70% more powerful than TNT. Used in the manufacture
of fuses for detonation and explosive specialties, including
the plastic explosive, Semtex, and in blasting caps. PETN
is also used as a medical vasodilator to lower blood pressure
by widening blood vessels to improve blood flow. PRTN
has been used in terrorism attempts in 2001 by the so-called
“shoe bomber,” in 2009 by the “underwear bomber,” and
most recently in October 2010, hidden in printer cartridges
being shipped internationally by passenger jet. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Shipping]
UN3344 Pentaerythrite tetranitrate mixture,
desensitized, solid, n.o.s. with >10% but not >20%
PETN, by mass, Hazard Class: 4.1; Labels: 4.1-Flammable
solid. UN0150 Pentaerythrite tetranitrate, wetted or
Pentaerythritol tetranitrate, wetted, or PETN, wetted or
Pentaerythrite tetranitrate, or Pentaerythritol tetranitrate
or PETN, desensitized, Hazard Class: 1D; Labels:1DExplosive
(with a mass explosion hazard); D-Substances
or articles which may mass detonate (with blast and/or
fragment hazard) when exposed to fire. | [Incompatibilities]
Treat PETN as an unstable explosive.
Rapid heating can cause detonation when heated to 210�C.
PETN is a dangerous high explosive and a strong oxidizer.
PETN normally requires a blasting cap or other kind of
detonator but may decompose explosively from concussion,
shock, friction, static charges. Keep away from combustible
materials; other oxidizers, for example, nitrates and
permanganates. Contact with sulfur trioxide may cause
detonation. Contact with reducing agents, e.g., zinc and
alkaline metals may cause explosion. May explode in
the presence of strong bases (i.e., sodium or potassium
hydroxide). May react with heavy metals. | [Waste Disposal]
Seek expert help with this
explosive material. Consult with environmental regulatory
agencies for guidance on acceptable disposal practices.
governing storage, transportation, treatment, and waste
disposal. | [Originator]
Pentanitrine, Promedica ,France,1948 | [Manufacturing Process]
Cooling water was turned on and 420 parts nitric acid of 94% strength was introduced into the nitrator. The amount of acid was such that the ratio of nitric acid to pentaerythritol was 4.29. The agitator was started and the agitator speed adjusted to 120 rpm. 92 parts pentaerythritol, which had been screened previously through a 14-mesh screen was used in each charge. About 45 parts pentaerythritol was added to the nitrator at such a rate that the temperature in the nitrator gradually rose to 110°F. This required about 12 minutes. Time was allowed for the temperature rise to cease before each succeeding increment of material was added.
After reaching 110°F the charge was maintained at about said temperature from 12 to 14 minutes during which time approximately 30 parts pentaerythritol was added to the nitrator. During the following 14 minutes, approximately, the remainder of the 92 parts pentaerythritol was added in like manner to the charge and the temperature gradually reduced. The pentaerythritol was introduced into the acid in finely divided and welldispersed particles and not in large unitary quantities. The entire 92 parts of pentaerythritol tetranitrate was introduced in 35 to 40 minutes. The pentaerythritol thus obtained was separated from the spent acid by filtering or drowning in water. To recover the spent acid the charge was passed onto a nutsch and filtered. The crude product was washed with water, then with a weak water-soluble alkali solution, such as sodium carbonate for example, and subsequently with water in order to remove the acid.
After the removal of acid, the nitrate was dried by suction on the nutsch for about 15 minutes. The dried material was refined by means of acetone treatment or other suitable refining means. About 210 parts refined pentaerythritol tetranitrate per charge was obtained. | [Brand name]
Pentritol Tempules
(Rhone-Poulenc Rorer); Peritrate (Parke-Davis). | [Therapeutic Function]
Coronary vasodilator | [Fire Hazard]
PETN is a high explosive, as powerful as cyclonite. It is more sensitive than TNT to shock. It explodes on percussion or heating. The detonating temperature is 210°C (410°F). The detonation velocity is 7.9 km/s. | [Synthesis]
Pentaerythritol tetranitrate, 2,2-bis(hydroxymethyl)-1,3-
propandioltetranitrate (19.1.2), is also synthesized by a nitration reaction of pentaerythritol with nitric acid, but using 2,2-bis(hydroxymethyl)-1,3-propandiol instead of glycerol as the starting material.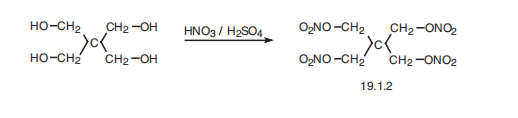
| [Purification Methods]
Crystallise pentaerythritol tetranitrate from acetone or acetone/EtOH. When crystallised from H2O at 0o, it may have m 26-28o (hydrate). It detonates more easily than TNT on percussion. The O-acetate, when crystallised from EtOH, has m 87-88o. Although it has been distilled at 60o/2mm, distillation should NOT be attempted as it is VERY EXPLOSIVE. Itis a vasodilator. [Marans et al. J Am Chem Soc 76 1304 1954, Camp et al. J Am Chem Soc 77 751 1955, Beilstein 1 IV 2816, 2 IV 264.] |
| Safety Data | Back Directory | [Hazard Codes ]
E,Xn | [Risk Statements ]
3-36-20/21/22-11 | [Safety Statements ]
35-36/37-26-16 | [RIDADR ]
0473 | [HazardClass ]
1.1A | [PackingGroup ]
II | [Safety Profile]
Human systemic effects by ingestion: dermatitis. Effects are sirmlar to those of nitroglycerin, i.e., headache, weakness, and fall in blood pressure. Very low oral toxicity. Severe explosion hazard when shocked or exposed to heat. It explodes at 215’C. On decomposition it emits hghly toxic fumes of NO,; can react vigorously with oxidizing materials. Used in detonators and explosive specialities. See also NITRATES and EXPLOSIVES, HIGH. | [Hazardous Substances Data]
78-11-5(Hazardous Substances Data) | [Toxicity]
LD50 oral in rat: 1660mg/kg |
|