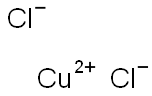Copper(II) chloride
- CAS No.
- 7447-39-4
- Chemical Name:
- Copper(II) chloride
- Synonyms
- CuCl2;COPPER CHLORIDE;Copper dichloride;Copper(II) chloride 97%;Copper(Ⅱ) chloride;dichlorocopper;Cupric(II) chloride;copper(ii)chloride(1:2);CUPRIC CHLORIDE, ANHYDROUSCUPRIC CHLORIDE, ANHYDROUSCUPRIC CHLORIDE, ANHYDROUSCUPRIC CHLORIDE, ANHYDROUS;Coclor
- CBNumber:
- CB1144723
- Molecular Formula:
- Cl2Cu
- Molecular Weight:
- 134.45
- MDL Number:
- MFCD00010972
- MOL File:
- 7447-39-4.mol
- MSDS File:
- SDS
| Melting point | 620 °C(lit.) |
|---|---|
| Boiling point | 993°C/760mmHg |
| Density | 3.386 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | powder |
| color | Yellow-brown |
| Specific Gravity | 3.386 |
| PH | 3.5 (50g/l, H2O, 20℃) |
| Water Solubility | 620 g/L (20 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,2633 |
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability | Stable. Reacts violently with potassium and sodium. Contact with acetylene may form explosive acetylides. Hygroscopic. |
| Surface tension | 72.7mN/m at 1.01g/L and 21℃ |
| CAS DataBase Reference | 7447-39-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | P484053J2Y |
| NIST Chemistry Reference | Copper chloride(7447-39-4) |
| EPA Substance Registry System | Cupric chloride (7447-39-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H315-H318-H410 | |||||||||
| Precautionary statements | P264-P273-P280-P301+P312-P302+P352+P312-P305+P351+P338 | |||||||||
| Hazard Codes | N,T,Xn,Xi | |||||||||
| Risk Statements | 52/53-50/53-36/37/38-25-22-51/53-41-23/24/25-50-36-21/22-38 | |||||||||
| Safety Statements | 61-57-45-37/39-29-26-60-36-44-36/37/39-28-39 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GL7000000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28274990 | |||||||||
| Toxicity | LD50 orally in Rabbit: 584 mg/kg | |||||||||
| NFPA 704 |
|
Copper(II) chloride price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.18247 | Copper(II) chloride forsynthesis | 7447-39-4 | 500g | $80.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 203149 | Copper(II) chloride 99.999% trace metals basis | 7447-39-4 | 10g | $117 | 2024-03-01 | Buy |
| Sigma-Aldrich | 203149 | Copper(II) chloride 99.999% trace metals basis | 7447-39-4 | 50g | $269.4 | 2024-03-01 | Buy |
| Alfa Aesar | 012457 | Copper(II) chloride, anhydrous, 98% min | 7447-39-4 | 50g | $32.8 | 2024-03-01 | Buy |
| Alfa Aesar | 012457 | Copper(II) chloride, anhydrous, 98% min | 7447-39-4 | 1kg | $140 | 2023-06-20 | Buy |
Copper(II) chloride Chemical Properties,Uses,Production
Physical Properties
Copper(II) chloride, CuCl2 is an anhydrous, brown solid copper salt which is soluble in water and gives a brownish aqueous solution when concentrated. When diluted, the solution changes its colour to green and then blue. CuCl2 is formed when copper(II) oxide, CuO, is treated with hydrochloric acid, HCl: CuO + 2HCI→CuCl2 + H2O.

The anhydrous form constitutes yellow to brown monoclinic crystals. It is hygroscopic; forms dihydrate on exposure to moist air; density 3.40 g/cm3; melts around 630°C with decomposition; soluble in water, ethanol and acetone.
The dihydrate exists as greenish blue orthorhombic crystals; density 2.51 g/cm3; decomposes at 100°C; is very soluble in water and ethanol (solubility greater than anhydrous salt in these solvents); also soluble in acetone; insoluble in ether.
Uses
Copper(II) chloride is used as a mordant in dyeing and printing of fabrics; as an ingredient of isomerization and cracking catalysts; and as a desulfurizing and deodorizing agent in petroleum industry. Other important applications are in copper plating of aluminum; in tinting-baths for iron and tin; in pigments for ceramics and glasses; as a fixer and desensitizer reagent in photography; in mercury extraction from ores; in laundry-marking and invisible inks; and in manufacture of several copper salts.
Preparation
Copper(II) chloride may be synthesized by heating elemental copper with chlorine:
Cu + Cl2 →CuCl2
Alternatively, it may be prepared by treating copper carbonate with hydrochloric acid followed by crystallization:
CuCO3 + 2HCl → CuCl2 + CO2 + H2O
In the above preparation, the hydrate of the salt crystallizes, precipitates, and may be dehydrated by heating under vacuum.
Reactions
When heated above 300°C, copper(II) chloride partially decomposes to copper( I) chloride and chlorine:
2CuCl2→ 2CuCl + Cl
Also, it is reduced to CuCl and elemental copper when treated with reducing agents.
Fluorination with fluorine produces copper(II) fluoride, CuF2. Adding potassium ferrocyanide to CuCl2 aqueous solution precipitates out reddish brown cupric ferrocyanide. Reaction with caustic soda forms blue cupric hydroxide:
CuCl2 + 2NaOH → Cu(OH)2 + 2NaCl
Black copper(II) sulfide, CuS, is obtained when hydrogen sulfide is passed through dissolved CuCl2.
CuCl2 forms several copper(II) complexes with several types of ligands in aqueous solutions.
Description
Copper chloride is a brownish-yellow powder.Molecular weight =134.44. Boiling point =993℃ (decomposes below this point); Freezing/Melting point =498℃.Hazard Identification (based on NFPA-704 M RatingSystem): Health 2, Flammability 0, Reactivity 0. Soluble inwater.
Chemical Properties
Cupric Chloride, brown-yellow powder, quite soluble in cold H2O or alcohol, very soluble in hot H2O. Catalyst for several organic syntheses, including production of vinyl chloride monomer.
Chemical Properties
Cupric chloride, CuCI2, also known as copper chloride, is a yellowish- brown solid that is soluble in water and alcohol. The dihydrate of cupric chloride, CUCI2·H20, is a green crystalline solid that is soluble in water. Cupric chloride is used in the textile industry as a mordant in the dyeing and printing of fabrics. Itis also used in refining gold,silver,and copper.
Uses
Supplement (trace mineral).
Uses
Isomerization and cracking catalyst, mordant in dyeing and printing fabrics, in electroplating baths for plating Cu on Al, in photography as a fixer, as a pigment for glass and ceramic.Copper(II) chloride is used as a co-catalyst with palladium(II) chloride used in the Wacker process to prepare ethanol from ethane. It acts as an effective catalyst for the tetrahydropyranylation of alcohols. It is involved in the production of vinyl chloride and dichloroethane as well as in the chlorination of aromatic hydrocarbons. It acts as deodorizing and desulfurizing agent in the petroleum industry, a mordant in textiles industry and in electrotyping baths for plating copper.
Uses
Definition
ChEBI: An inorganic chloride of copper in which the metal is in the +2 oxidation state.
General Description
Cupric chloride and ammonia contains various concentrations of cupric chloride in ammonium hydroxide. This forms a copper ammonia complex. Cupric chloride is corrosive to skin, eyes, mucous membranes and metal. Cupric chloride is used to etch copper from printed circuit boards.
Reactivity Profile
CUPRIC CHLORIDE has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by intravenous and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. See also COPPER COMPOUNDS and CHLORIDES. Can react violently with K and Na. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
Copper chloride is used in petroleum,textiles, metallurgy, photography, agricultural products,feed additives, and wood preservation. It is also used inlight-sensitive paper manufacturing, glass pigments, ceramics, and in making cyclonitrile.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemicalhas been swallowed, get medical attention. If victim isconscious, administer water or milk. Do not inducevomiting.
storage
Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Prior toworking with this chemical you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from incompatiblematerials listed above, moisture, and heat.
Shipping
UN2802 Copper chloride, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crystallise the chloride from hot dilute aqueous HCl (0.6mL/g) by cooling in a CaCl2-ice bath. It is dehydrated by heating on a steambath under vacuum. It is deliquescent in moist air but efflorescent in dry air. The dihydrate is emerald green but blue when free from solvent. Concentrated solutions are yellow-green in colour but are blue when free from solvent. Concentrated solutions are yellow-green and become yellow on adding conc HCl. A very dilute solution is pure blue due to Cu(H2O)42+ [Donan & Bassett J Chem Soc 81 939 1902.]. CuCl2 is very deliquescent and is soluble in MeOH or EtOH to give green crystals of Cu(ROH)2Cl2. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1008 1965.]
Incompatibilities
Contact with strong acids forms monovalent copper salts and toxic hydrogen chloride gas. Forms shock-sensitive and explosive compounds with potassium, sodium, sodium hypobromite, nitromethane, acetylene. Keep away from moisture and alkali metals. Attacks metals in the presence of moisture. Reacts with moist air to form cupric chloride dihydrate. May attack some metals, paints, and coatings. May be able to ignite combustible materials.
Copper(II) chloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
View Lastest Price from Copper(II) chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-09 | Copper(II) chloride
7447-39-4
|
US $0.00-0.00 / kg | 1kg | 0.99 | 100tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Copper(II) chloride
7447-39-4
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-06-20 | Copper(II) chloride
7447-39-4
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Copper(II) chloride
7447-39-4
- US $0.00-0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Copper(II) chloride
7447-39-4
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Copper(II) chloride
7447-39-4
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
7447-39-4(Copper(II) chloride)Related Search:
1of4