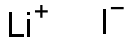Lithium iodide
- CAS No.
- 10377-51-2
- Chemical Name:
- Lithium iodide
- Synonyms
- LiI;Lithiumiodid;Lithium iodide (LiI);Tenephrol;Lithiumjodid;ithium iodide;LITHIUM IODIDE;Iodide lithiuM;LITHIUM IODIDE;lithiumiodide(bpc)
- CBNumber:
- CB8688141
- Molecular Formula:
- ILi
- Molecular Weight:
- 133.85
- MDL Number:
- MFCD00011092
- MOL File:
- 10377-51-2.mol
- MSDS File:
- SDS
| Melting point | 446 °C(lit.) |
|---|---|
| Boiling point | 1171 °C |
| Density | 3.49 g/mL at 25 °C(lit.) |
| refractive index | 1.955 |
| Flash point | 1170-1190°C |
| storage temp. | Store below +30°C. |
| solubility | 1640g/l soluble |
| form | powder |
| color | White to yellow to beige |
| Specific Gravity | 4.076 |
| Water Solubility | Soluble in water, ethanol and acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,5535 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Stability | hygroscopic |
| CAS DataBase Reference | 10377-51-2(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | LITHIUM IODIDE |
| FDA UNII | S6K2XET783 |
| EPA Substance Registry System | Lithium iodide (10377-51-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280a-P305+P351+P338-P321-P332+P313-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/38-36/37/38-22 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| WGK Germany | 1 | |||||||||
| F | 3-8-10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2827 60 00 | |||||||||
| NFPA 704 |
|
Lithium iodide price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.18287 | Lithium iodide anhydrous for synthesis | 10377-51-2 | 2g | $111 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18287 | Lithium iodide anhydrous for synthesis | 10377-51-2 | 50g | $210 | 2024-03-01 | Buy |
| Sigma-Aldrich | 218219 | Lithium iodide beads, 99% | 10377-51-2 | 10g | $139 | 2024-03-01 | Buy |
| Sigma-Aldrich | 218219 | Lithium iodide beads, 99% | 10377-51-2 | 50g | $381 | 2024-03-01 | Buy |
| Alfa Aesar | 013600 | Lithium iodide, ultra dry, 99.999% (metals basis) | 10377-51-2 | 25g | $342 | 2024-03-01 | Buy |
Lithium iodide Chemical Properties,Uses,Production
Description
Lithium iodide (chemical formula: LiI) is the compound of lithium and iodine. It can be used as an electrolyte in high-temperature batteries, long-life batteries which is required, e.g. by artificial pacemaker as well as in the electrolyte of dye-sensitized solar cells. In organic synthesis, it can be used for C-O bonds cleavage such as converting methyl esters to carboxylic acids. In addition, it can be used as a radio contrast agent for X-ray computed tomography imaging studies.

Physical properties
Lithium iodide, a white crystalline solid, is deliquescent as are lithium chloride and lithium bromide. Lithium iodide is readily oxidized in air to yield iodine which discolors the crystals.
Lithium iodide is very soluble in water and forms the following hydrates: Lii-0.5 H2O, L i l·O , LiI-2H20 and LiI·3H20. The hydrates have congruent melting points unlike the hydrates of the chloride and the bromide.
Lithium iodide is even more soluble in organic solvents than the chloride or the bromide.
Uses
Lithium iodide can be used for the catalytic dehydrogenation of hydrocarbons. Oxidation of lithium iodide yields iodine which reacts with the hydrocarbon to form an organic iodide. The intermediate is dehydroiodinated to yield an olefin.For example, butane may be converted to butadiene.
Preparation
The laboratory preparation of lithium iodide is carried out in much the same way as the industrial preparation.
Lithium iodide is prepared by the reaction of lithium hydroxide monohydrate or lithium carbonate with hydriodic acid, which may be prepared in situ by the reduction of iodine. The iodide is usually not isolated as the anhydrous material but is used as a solution or as the solid lithium iodide trihydrate.
Hydriodic acid may be prepared by the reaction of hydrogen sulfide and iodine. The hydriodic acid solution must be kept under an inert atmosphere to prevent air oxidation to iodine. After combination of the acid with the lithium compounds, the solution may be evaporated to yield lithium iodide trihydrate, LiI· 3H20. The anhydrous material may be made by extremely careful dehydration of the trihydrate under vacuum and with slow heating. The lithium iodide or its trihydrate must be handled under an inert atmosphere to avoid oxidation of the iodide ion to iodine.
References
http://en.wikipedia.org/wiki/Lithium_iodide
Chemical Properties
White to off-white crystalline powder
Physical properties
White cubic crystals; refractive index 1.955; density 4.076 g/cm3; melts at 449°C; vaporizes around 1,180°C; highly soluble in water (165 g/100g at 20°C), solubility greatly increases in hot water (433g/100g at 80°C); also very soluble in methanol (343 g/100g at 20°C) and ammonia; soluble in acetone (42.6g/100g at 18°C).
The trihydrate, LiI?3H2O, is a yellowish solid (due to the release of iodine when exposed to air); hexagonal crystals; hygroscopic; density 3.48 g/cm3; loses iodine when heated in air; loses one molecule of water of crystallization at 73°C becoming dihydrate, LiI?2H2O and loses the second molecule at 80°C, forming monohydrate, LiI?H2O and becomes anhydrous at 130°C; highly soluble in water; soluble in ethanol and acetone.
Uses
Controls regioselectivity in palladium-catalyzed allylic alkylation reactions.
Uses
It is used as a absorbent in refrigeration process, as a catalyst, and in photography. It is also useful for organic synthesis, pessat iodination, cleavage of esters, ethers, epoxides, decarboethoxylation, carbonylation, and aldol condensation. It is used for manufacturing medicines and mineral waters. It is used in electochemicstry and redox reactions. Lithium iodide is also a catalyzed conjugate addition reaction of β-dicarbonyl compounds. It is also useful for tetrathioquinodimethane.
Uses
Controls regioselectivity in palladium catalyzed allylic alkylation reactions.1
Preparation
The trihydrate salt is obtained by neutralization of lithium hydroxide or lithium carbonate solution with pure hydriodic acid followed by concentration of the solution for crystallization:
LiOH + HI → LiI + H2O
When heated in a vacuum, the trihydrate dehydrates to anhydrous salt.
Purification Methods
Crystallise it from hot water (0.5mL/g) by cooling in a CaCl2-ice EtOH or from an acetone-Dry-Ice bath. Dry it under a vacuum over P2O5 for 1hour at 60o and then at 120o. It is deliquescent and should be stored in a tightly stoppered vessel in the dark.
Lithium iodide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Huaran Industrial Co.,Ltd | 17749774353 | 1715438216@qq.com | China | 52 | 58 |
| Shijiazhuang Jianxin Biotechnology Co., LTD | +undefined+86-17692708569 | liyingjian1997@gmail.com | China | 787 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 | hjt@hong-jin.com | China | 228 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
Related Qustion
- Q:Is lithium iodide ionic?
- A:We should expect that even if LiI is ionic, it has a high proportion of covalent properties.
- Apr 22,2024
View Lastest Price from Lithium iodide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-16 | Lithium iodide
10377-51-2
|
US $300.00-250.00 / kg | 1kg | 99.9% | 500KG/Month | Shijiazhuang Jianxin Biotechnology Co., LTD | |
 |
2023-09-23 | Lithium iodide
10377-51-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-09-06 | Lithium iodide
10377-51-2
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Lithium iodide
10377-51-2
- US $300.00-250.00 / kg
- 99.9%
- Shijiazhuang Jianxin Biotechnology Co., LTD
-

- Lithium iodide
10377-51-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Lithium iodide
10377-51-2
- US $0.00-0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.