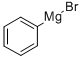PHENYLMAGNESIUM BROMIDE
- CAS No.
- 100-58-3
- Chemical Name:
- PHENYLMAGNESIUM BROMIDE
- Synonyms
- PhMgBr;Phenylmagnesi;nesium bromide;bromophenylmagnesium;Phenylbromomagnesium;bromophenyl-magnesiu;(Bromomagnesio)benzene;PhenylMagnesiuM BroMid;Magnesium, bromophenyl-;PHENYLMAGNESIUM BROMIDE
- CBNumber:
- CB1153390
- Molecular Formula:
- C6H5BrMg
- Molecular Weight:
- 181.31
- MDL Number:
- MFCD00000038
- MOL File:
- 100-58-3.mol
| Melting point | 153-154 °C |
|---|---|
| Density | 1.134 g/mL at 25 °C |
| Flash point | −40 °F |
| storage temp. | water-free area |
| solubility | Miscible with tetrahydrofuran. |
| form | Liquid |
| color | Yellow to brown |
| Specific Gravity | 1.14 |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| BRN | 3588849 |
| InChIKey | ANRQGKOBLBYXFM-UHFFFAOYSA-M |
| CAS DataBase Reference | 100-58-3(CAS DataBase Reference) |
| EPA Substance Registry System | Magnesium, bromophenyl- (100-58-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302-H314 | |||||||||
| Precautionary statements | P210-P233-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | F+,C,F | |||||||||
| Risk Statements | 12-14-22-34-19-11-67-40-37 | |||||||||
| Safety Statements | 16-26-36/37/39-45-33-29-7/8-6A-43B | |||||||||
| RIDADR | UN 3399 4.3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| F | 1-3-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
PHENYLMAGNESIUM BROMIDE price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 331376 | Phenylmagnesium bromide solution 1.0?M in THF | 100-58-3 | 4X25ML | $87 | 2024-03-01 | Buy |
| Sigma-Aldrich | 171565 | Phenylmagnesium bromide solution 3.0M in diethyl ether | 100-58-3 | 50ml | $72.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 171565 | Phenylmagnesium bromide solution 3.0?M in diethyl ether | 100-58-3 | 4X25ML | $95 | 2024-03-01 | Buy |
| Sigma-Aldrich | 171565 | Phenylmagnesium bromide solution 3.0M in diethyl ether | 100-58-3 | 18l | $3280 | 2024-03-01 | Buy |
| TCI Chemical | P2025 | Phenylmagnesium Bromide (16% in Tetrahydrofuran, ca. 1mol/L) | 100-58-3 | 250g | $153 | 2024-03-01 | Buy |
PHENYLMAGNESIUM BROMIDE Chemical Properties,Uses,Production
Reduction agent for preparation of metal carbonyl compound
In 1890, L.Mond found that when carbon monoxide was burned after being put through the active metal nickel powder, it emitted green light flame. After the resulting gas was cooled, he could obtain colorless liquid (melting point 298K, the boiling point of 316K); if this gas is flowed through a heated glass tube, then it can be seen that the metallic nickel was deposited on the wall. This gas is tetracarbonyl nickel Ni(CO)4. Since the 1960s, people have synthesized a hundreds kinds of such carbonyl compounds and their derivatives. Almost all of the transition metal can form such compounds. This kind of special complex formed through transition metal with carbon monoxide ligands is called metal carbonyls, otherwise known as carbonyl complexes. Metal carbonyl occupies an important position in both theoretical research and practical application in modern inorganic chemistry.
Preparation of metal carbonyl compound can usually via the following methods:
1. Direct synthesis. Most metal carbonyl complexes are prepared by direct combination between metal and carbon monoxide. However, the metal must be new reduction products, and is in a very activated state.
2. Reduction carbonylation effect at high pressure. Apply reducing agent under high pressure to enable the carbonylation reaction between the metal and carbonyl group with the major reducing agents mainly including hydrogen, active metals, phenylmagnesium bromide (C6H5MgBr) and so on.
3. With thermal decomposition or UV irradiation decomposition, we can obtain certain polynuclear carbonyl compounds.
4. Two different kinds of metal carbonyl compounds can interact with each other to give hetero-nuclear carbonyl compound.
Related chemical reaction
1. Phenylmagnesium bromide can react with ferric chloride to give the coupling product. Cyclopentadienyl magnesium bromide can react with ferric chloride to give ferrocene.
2. It can react with chromium trichloride in diethyl ether solution to generate dibenzenechromium.
3. Hexaphenyldilead can be used as the antioxidant of polyphenylene ether lubricant that can be produced from the reaction of lead chloride with phenyl lithium or phenyl magnesium bromide.
4. Take tetrahydrofuran as the solvent, have phenylmagnesium bromide reacted with germanium tetrachloride to obtain tetraphenyl germane.
5. It can be obtained through the low-temperature reaction between lead chloride and phenylmagnesium bromide (Or phenyllithium) in the presence of iodobenzene to derive tetraphenyl lead.
6. Sodium tetraphenylborate is mainly used for the determination of potassium ion, ammonium ion, rubidium ion and cesium ion. It can be obtained with the following process: phenylmagnesium bromide is interacted with boron trifluoride/diethyl ether to generate Tetraphenylboron magnesium bromide first. Evaporate the diethyl ether; add water and then used the calculated amount of sodium carbonate for treatment to obtain it.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Chemical Properties
Clear light brown to brown solution when properly
Uses
Grignard reagent in greener solvent, 2-methyltetrahydrofuran (2-MeTHF)
2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry
Uses
Phenylmagnesium Bromide is a reagent used in the preparation of arylcarbonimidic dichlorides via dichloroiodobenzene-mediated gem-dichlorination of aryl isonitriles.
Definition
ChEBI: Phenylmagnesium bromide is an arylmagnesium halide. It has a role as a Grignard reagent.
PHENYLMAGNESIUM BROMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5866 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9355 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32836 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
View Lastest Price from PHENYLMAGNESIUM BROMIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-08-22 | PHENYLMAGNESIUM BROMIDE
100-58-3
|
US $10.00 / KG | 1KG | 99.% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-01-18 | phenylmagnesium bromide
100-58-3
|
US $16.00 / KG | 1600KG | 99% | 20T | Xiamen Wonderful Bio Technology Co., Ltd. | |
 |
2023-07-19 | phenylmagnesium bromide
100-58-3
|
US $30.00 / KG | 1KG | 99% | 20 Tons | Wuhan wingroup Pharmaceutical Co., Ltd |
-

- PHENYLMAGNESIUM BROMIDE
100-58-3
- US $10.00 / KG
- 99.%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- phenylmagnesium bromide
100-58-3
- US $16.00 / KG
- 99%
- Xiamen Wonderful Bio Technology Co., Ltd.
-

- phenylmagnesium bromide
100-58-3
- US $30.00 / KG
- 99%
- Wuhan wingroup Pharmaceutical Co., Ltd
100-58-3(PHENYLMAGNESIUM BROMIDE)Related Search:
1of4