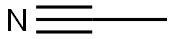Acetonitrile
- CAS No.
- 75-05-8
- Chemical Name:
- Acetonitrile
- Synonyms
- ACN;MeCN;CH3CN;AN;Acetonitril;MGDA;anhydrous Acetonitrile;ETHANENITRILE;Cyanomethan;MOBILE PHASE ACETONITRILE
- CBNumber:
- CB2127174
- Molecular Formula:
- C2H3N
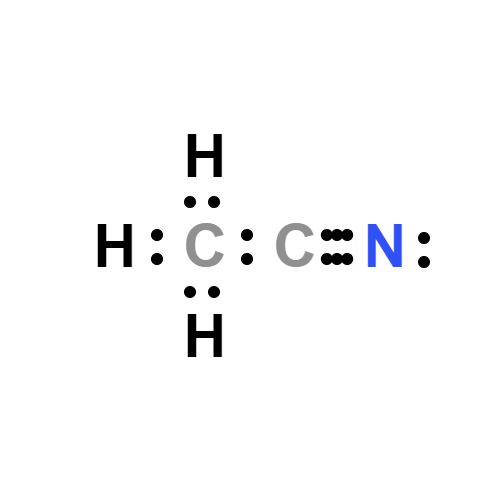
Lewis structure
- Molecular Weight:
- 41.05
- MDL Number:
- MFCD00001878
- MOL File:
- 75-05-8.mol
- MSDS File:
- SDS
| Melting point | ?45 °C (lit.) |
|---|---|
| Boiling point | 81-82 °C (lit.) |
| Density | 0.786 g/mL at 25 °C (lit.) |
| vapor density | 1.41 (vs air) |
| vapor pressure | 72.8 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 48 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | organic solvents: soluble(lit.) |
| pka | 25(at 25℃) |
| form | liquid |
| color | <10(APHA) |
| Specific Gravity | approximate 0.78(20/20℃) |
| Odor | Aromatic ether-like odor detectable at 40 ppm |
| Relative polarity | 0.46 |
| explosive limit | 3.0-17%(V) |
| Odor Threshold | 13ppm |
| Water Solubility | miscible |
| λmax |
λ: 195 nm Amax: ≤0.12 λ: 200 nm Amax: ≤0.032 λ: 230 nm Amax: ≤0.0044 λ: 235 nm Amax: ≤0.0044 λ: 250 nm Amax: ≤0.0044 λ: 400 nm Amax: ≤0.0044 |
| Merck | 14,70 |
| BRN | 741857 |
| Henry's Law Constant | 7.30 at 5 °C, 8.90 at 10 °C, 11.6 at 15 °C, 14.6 at 20 °C, 17.6 at 25 °C (headspace-GC, Ji and Evans, 2007) |
| Exposure limits | TLV-TWA 70 mg/m3 (40 ppm) (ACGIH and OSHA); STEL 105 mg/m3 (60 ppm) (ACGIH); IDLH 4000 ppm (NIOSH). |
| Dielectric constant | 37.5(21℃) |
| Stability | Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. |
| LogP | -0.340 |
| FDA 21 CFR | 514.1 |
| CAS DataBase Reference | 75-05-8(CAS DataBase Reference) |
| EWG's Food Scores | 4-7 |
| FDA UNII | Z072SB282N |
| NIST Chemistry Reference | Acetonitrile(75-05-8) |
| EPA Substance Registry System | Acetonitrile (75-05-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H312+H332-H319 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi,Xn,T | |||||||||
| Risk Statements | 11-36-20/21/22-10-36/37/38-23/24/25-41-24-20/22 | |||||||||
| Safety Statements | 16-36/37-45-36/37/39-27-26-36 | |||||||||
| RIDADR | UN 1993 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | AL7700000 | |||||||||
| F | 9 | |||||||||
| Autoignition Temperature | 524 °C | |||||||||
| Hazard Note | Highly Flammable/Harmful/Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29269095 | |||||||||
| Toxicity | LD50 orally in rats: 3800 mg/kg (Smyth) | |||||||||
| IDLA | 137 ppm | |||||||||
| NFPA 704 |
|
Acetonitrile price More Price(237)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | TX1277 | Acetonitrile/TFA 0.1% Mobile Phase | 75-05-8 | 4L | $731 | 2024-03-01 | Buy |
| Sigma-Aldrich | TX1278 | Acetonitrile/TFA 0.05% Mobile Phase | 75-05-8 | 20L | $2760 | 2024-03-01 | Buy |
| Sigma-Aldrich | TX1277 | Acetonitrile/TFA 0.1% Mobile Phase | 75-05-8 | 20L | $2760 | 2024-03-01 | Buy |
| Sigma-Aldrich | FX0437 | Acetonitrile/Formic Acid 0.1% Mobile Phase | 75-05-8 | 4L | $768 | 2024-03-01 | Buy |
| Sigma-Aldrich | FX0437 | Acetonitrile/Formic Acid 0.1% Mobile Phase | 75-05-8 | 20L | $2920 | 2024-03-01 | Buy |
Acetonitrile Chemical Properties,Uses,Production
The simplest organic nitrile
Acetonitrile is the simplest organic nitrile, usually also called as nitrile methyl cyanide and methane. It is a colorless transparent liquid at room temperature. It is highly volatile, with special smell like ether, and flammable with flame burning brightly. It is mutually soluble in water, methanol, carbon tetrachloride, methyl acetate, ethyl acetate, ethylene dichloride, and many other non-saturated hydrocarbon solvents. It is toxic and can be metabolized into hydrogen cyanide and thiocyanate. Acetonitrile is a good solvent with excellent performance and is an important organic intermediate. It is also widely used as a polar aprotic solvent. The biggest application of acetonitrile is as a solvent which can be used as the solvents for the synthesis of vitamin A, cortisone, carbon amine drugs and their intermediates solvent. It also used as an active medium solvent in the manufacture of vitamin B1 and amino acids. It can substitute chlorinated solvents as a vinyl coating, an extracting agent of fatty acid, a alcohol denaturant, the extracting agent of butadiene, and the solvent of acrylonitrile synthetic fibers. It also has a lot of applications in fabric dyeing, light industry, spice manufacturing, and photographic materials manufacturing.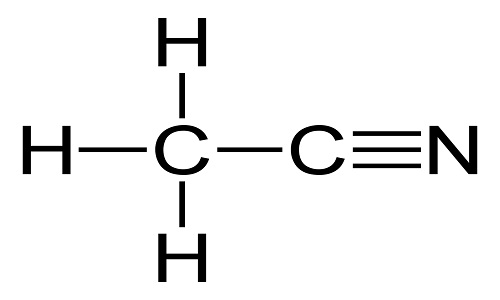
Acetonitrile Structure
Toxic and hazardous effects
Acetonitrile class is produced by heating a mixture of glacial acetic acid and acetamide. It is an important industrial solvent primarily used for the medium of organic synthesis (e.g. acetophenone, 1-naphthyl acetic acid, thiamine, etc.), extracting agent of fatty acids, and alcohol denaturant. During the production process, exposure to liquid or vapor may cause poisoning.
[Clinical manifestations] Acute and occupational acetonitrile poisoning is not uncommon. There are many reports at both home and abroad. Vapor of acetonitrile has mild irritation so it can cause some degree of upper respiratory tract irritation in the case of high concentrations. Compared with hydrogen cyanide, acetonitrile although causes symptoms like nausea, vomiting, abdominal pain, diarrhea, chest pain, fatigue, and weakness, even respiratory depression in severe case, sometimes also causes hypotension, coma, and convulsions, but its onset process is relatively slow with the incubation period over 4H; nor does it cause illness as severe as hydrogen cyanide. It also rarely causes sudden death; For poisoned patients, their heart rates, pulse rates as well as the respiration rates decrease. They often got pale faces and also suffer kidney impairment like protein-urine. The toxicity of acetonitrile is not only related to the released CN-in vivo but also related to itself and its thiocyanate metabolites. There are currently no clinic products for treating chronic acetonitrile poisoning.
[Diagnosis and differential diagnosis] Diagnosis is mainly based on reliable history of exposure to large doses of acetonitrile and clinical characteristics, the appearance of similar poisoning effects for mutual contractees plays a obvious indication role; timely determination of plasma CN-, SCN-, and acetonitrile content is also indicative, and is the biomarker of contacting with acetonitrile. However, it cannot tell the existence and extent of poisoning. Acute acetonitrile poisoning should be paid attention to distinguish with toxic poisoning caused by other industrial toxic substance such as organic solvents, asphyxiating gas. It should also be distinguished from cerebrovascular accident, diabetic coma.
[Treatment] Refer to the content on treatment of hydrogen cyanide but cut the dose of methemoglobin forming agent by half. In the presence of sodium thiosulfate, we can apply in early phase of the slowly acted methemoglobin generation agents such as amino benzene acetone (PAPP). Taken one orally each time, and can repeat for every 4H. For the next day, maintaining with sodium thiosulfate is enough. The dosage of sodium thiosulfate can also be cut by half two days later and totally stopped after 3 to 5 days.
Because of the toxic effect of the acetonitrile, when apply it as the antidote of cyanide antidote, people should be particularly participate in actively supportive treatment according to the symptomatic and supportive treatment, pay attention to the function maintenance of the heart, lung, brain, and apply rehydration for diuresis to accelerate the toxic discharge and reduce kidney impairment.
Purification methods
Industrially, acetonitrile is a byproduct of the reaction between propylene and ammonia which produces acrylonitrile, so often acetonitrile often contains water, acrylonitrile, ether, ammonia and some other impurities, even hydrolyzed acetic acid and ammonia. The purification method is as below:
1. Add phosphorus pentoxide (10-20g/L) into acetonitrile; heat and reflux until reaching colorless which can remove most water; avoid adding an excess of phosphorus pentoxide which will generate an orange polymer. Add a small amount of potassium carbonate into the distilled acetonitrile and continue distillation which can further remove excess phosphorus pentoxide; finally fractionate by fractional distillation column.
2. Use 36 g of mashed potassium permanganate and 28 g of mashed potassium carbonate to reflux 1L common anhydrous acetonitrile for 5 hours before evaporate it. Then add 10g of phosphorus pentoxide to the evaporated solvent; reflux for another 5 hours, fine slip, keeping the temperature constant, take the fraction of 81 °C.
3. Adding 4A molecular sieves or silica gel and shaking can also remove most of the water in acetonitrile. Next, stir it together with the calcium hydroxide until no hydrogen being further released; fractionate to get acetonitrile which also contain only a small amount of water without the existence of any acetate.
4. Acetonitrile can also be mixed together with methylene chloride, benzene and trichlorethylene for azeotropic distillation and drying.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Laboratory use
Acetonitrile is also used as a polar aprotic solvent.
In inorganic chemistry, acetonitrile is widely used as a ligand which is abbreviated MeCN. For example, acetonitrile complex PdCl2 (MeCN)2 can be produced by thermal polymerization of palladium chloride in the suspension of acetonitrile.
The high dielectric constant of acetonitrile makes it a popular cyclic voltammetry of solvents. Acetonitrile can also be used as a two-carbon raw material in organic synthesis. It can produce malononitrile via reaction with cyanogen chloride.
Acetonitrile can also be used as the mobile phase molecules which are commonly used in the column chromatography, more modernized high performance liquid chromatography (HPLC).
In the field of nuclear medicine, acetonitrile is used for the synthesis of radiopharmaceutical like fluoro-deoxy-glucose positron (FDG). During the synthesis of FDG, the evaporation of acetonitrile can take away the water in the reaction system. The exact content of acetonitrile in the reaction system plays a significant role in ensuring the synthesis efficiency and quality of medicines; at the same time, acetonitrile is also sued as the solvent and the matrix for the reaction system. In addition, in the routine quality inspection of FDG, acetonitrile: water mixture (for example, 85% v/v) is also applied as the mobile phase of TLC.
Uses
Acetonitrile is the raw material for preparing orthoacetate. It is also used as the intermediate of producing DV-acid methyl ester and 2-chloro-3,3,3-trifluoro-1-propenyl-2,2-dimethyl cyclopropanecarboxylate. It can also be used as the raw materials of making pyrimidine derivatives which is the intermediate of sulfonylurea herbicides. Moreover, it can be used for making vitamin B1 in the field of pharmaceutical industry and as the extraction agent of C4 fraction in the synthetic rubber industry.
Used as nitrile rubber monomer; Used for pharmaceutical industry and extraction of carbon IV.
As standard reference in chromatographic analysis; also as solvent and stationary phase for gas chromatography.
The major application of acetonitrile is as a solvent such as solvents for butadiene extraction, solvent for synthetic fibers and solvents for some special paints. In the oil industry, acetonitrile is used as the solvent for removing tar, phenol and other substances from petroleum hydrocarbons. It is also used as the solvent for extracting fatty acids from vegetable and animal oil in the fatty acid industry, and used as the reaction medium of the recrystallization of steroidal drugs in medicine industry. The binary azeotropic mixtures of acetonitrile and water are often used when a polar solvent of high dielectric constant is demanded: containing 84% acetonitrile, boiling point: 76 °C. Acetonitrile is used as the intermediate of pharmaceutical (vitamin B1) and spices, as the raw materials for making the synergist of triazine nitrogenous fertilizer, and also as a denaturant for ethyl alcohol. Moreover, it can also be used for synthesizing ethylamine, acetic acid, etc., and have many applications in textile dyeing and light industry.
It is used as the solvent of most inorganic compounds. It is also used as the solvent for spectrophotometric measurement, as a non-aqueous solvent, and as the diluents for determination of the carboxyl group. Furthermore, it is also applied in recrystallization of steroids and extraction of fatty acid, and also used as the solvents of High pressure liquid chromatography (HPLC).
Production method
There are many ways of making acetonitrile. Those major ways for industrial production include acetate amination method, acetylene amination method and propylene ammoxidation byproduct method. 1. Acetate amination method use acetate and ammonia as raw materials with reaction being performed at a temperature of 360-420 °C in the presence of aluminum oxide as the catalyst. This is a one-step synthesis method. The reaction mixture is further gone through water absorption and fine distillation to get the final product. Material consumption quantity: acetate (98%) 1763kg /t, ammonia (99.5%) 691kg/t. 2. Acetylene amination method uses ammonia and acetylene as the raw materials and the reaction is carried out at a temperature of 500-600 °C with aluminum oxide being the catalyst. It is again a one-step synthesis approach. Material consumption quantity: acetylene 10231 m3, ammonia (99.4%) 1007 kg/t. 3. Propylene amination and oxidation byproduct method use propylene, ammonia, and air as the raw materials. It produces acrylonitrile with the catalyst while producing acetonitrile as byproducts. Per ton of acrylonitrile can make 25-100kg byproduct of acetonitrile. 4. Made from the dehydration reaction between acetamide and phosphorus pentoxide. 5. Obtained from reaction between dimethyl sulfate and sodium cyanide.
Acetonitrile is usually the byproduct of ammoxidation reaction used for producing acrylonitrile. We can also apply acetate amination method with aluminum oxide as the catalyst. Acetonitrile is obtained by one-step reaction at 360 °C. Reaction equation:
CH3COOH + NH3 [Al2O3] → CH3CN + 2H2O.
Description
Acetonitrile is a liquid with an etherlike odor. It is a highly polar, volatile solvent used in many different industrial applications. It is widely used in the pharmaceutical, photographic, chemical, and analytical industries. It is useful as an industrial solvent for the separation of olefins, polymers, spinning fibers, and plastics. Other uses include the extraction and refining of copper and by-product ammonium sulfate; used for dyeing textiles and in coating compositions; used as a stabilizer for chlorinated solvents; manufacture of perfumes and cosmetics; and as a general reagent in a wide variety of chemical processes.
Chemical Properties
Acetonitrile (methyl cyanide), CH3CN, is a colorless liquid with a sweet, ethereal odor. It is completely miscible with water and its high dielectric strength and dipole moment make it an excellent solvent for both inorganic and organic compounds including polymers. it is commonly applied to the development and manufacturing of cosmetics, pharmaceutical and agricultural products.Acetonitrile has been banned in cosmetic products in the European Economic Area (EEA) since early 2000 and acetone and ethyl are often preferred as safer for domestic use.
Physical properties
Colorless liquid with an ether-like or pungent odor of vinegar. A detection odor threshold concentration of 1,950 mg/m3 (1,161 ppmv) was experimentally determined by Dravnieks (1974). An odor threshold concentration of 13 ppmv was reported by Nagata and Takeuchi (1990).
Uses
Acetonitrile is the simplest organic nitrile. It is a by-product of the manufacture of acrylonitrile, and acetonitrile has, in fact, replaced acrylonitrile. Acetonitrile has a number of uses, primarily as an extraction solvent for butadiene; as a chemical interme- diate in pesticide manufacturing; as a solvent for both inorganic and organic compounds; to remove tars, phenols, and coloring matter from petroleum hydrocarbons not soluble in acetonitrile; in the production of acrylic fi bers; in pharmaceuticals, perfumes, nitrile rubber, and acrylonitrile-butadiene-styrene (ABS) resins; in high-performance liquid and gas chro- matographic analysis; and in extraction and refi ning of copper. It is used as a starting material for the produc- tion of acetophenone, alpha-naphthalenacetic acid, thiamine, and acetamidine.
Application
Acetonitrile is used as a solvent for polymers, spinning fibers, casting and molding plastics, and HPLC analyses; for extraction of butadiene and other olefins from hydrocarbon streams; in dyeing and coating textiles; and as a stabilizer for chlorinated solvents. It occurs in coal tar and forms as a by-product when acrylonitrile is made. Although acetonitrile is one of the more stable nitriles, it undergoes typical nitrile reactions and is used to produce many types of nitrogencontaining compounds.Acetonitrile also is used as a catalyst and as an ingredient in transitionmetal complex catalysts.
Definition
ChEBI: Acetonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by a methyl group. It has a role as a polar aprotic solvent and an EC 3.5.1.4 (amidase) inhibitor. It is an aliphatic nitrile and a volatile organic compound.
Production Methods
Acetonitrile is mainly prepared by dehydration of acetamide (CH3CONH2) with glacial acetic acid (Turner 1950) or by reacting acetic acid with ammonia at 400-500°C in the presence of a dehydration catalyst (Anon 1978).
General Description
A colorless limpid liquid with an aromatic odor. Flash point 42°F. Density 0.783 c / cm3. Toxic by skin absorption. Less dense than water. Vapors are denser than air.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Acetonitrile decomposes when heated to produce deadly toxic hydrogen cyanide gas and oxides of nitrogen. Strongly reactive [Hawley]. May react vigorously with strong oxidizing reagents, sulfuric acid, chlorosulfonic acid, sulfur trioxide, perchlorates, nitrating reagents, and nitric acid. [Sax, 9th ed., 1996, p. 20]. Potentially explosive in contact with nitrogen-fluorine compounds (e.g., tetrafluorourea) [Fraser, G. W. et al., Chem. Comm., 1966, p. 532].
Health Hazard
Acetonitrile liquid or vapor is irritating to the skin, eyes, and respiratory tract. Acetonitrile has only a modest toxicity, but it can be metabolized in the body to hydrogen cyanide and thiocyanate. Acetonitrile causes delayed symptoms of poisoning (several hours after the exposure) that include, but are not limited to, salivation, nausea, vomiting, anxiety, confusion, hyperpnea, dyspnea, respiratory distress, disturbed pulse rate, unconscious- ness, convulsions, and coma. Cases of acetonitrile poisoning in humans (or, more strictly, of cyanide poisoning after exposure to acetonitrile) are rare but not unknown, by inha- lation, ingestion, and (possibly) by skin absorption. Repeated exposure to acetonitrile may cause headache, anorexia, dizziness, weakness, and macular, papular, or vesicular dermatitis.
Flammability and Explosibility
Acetonitrile is a flammable liquid (NFPA rating = 3), and its vapor can travel a
considerable distance to an ignition source and "flash back." Acetonitrile vapor
forms explosive mixtures with air at concentrations of 4 to 16% (by volume).
Hazardous gases produced in a fire include hydrogen cyanide, carbon monoxide,
carbon dioxide, and oxides of nitrogen. Carbon dioxide or dry chemical
extinguishers should be used for acetonitrile fires.
Industrial uses
Acetonitrile is used as a solvent both in industry and in the laboratory, as a rodenticide, and in the denaturation of alcohol. Because of both its solvent properties and volatility, it is useful for extracting vegetable and animal oils and dissolving hydrocarbons, oils, and greases. Acetonitrile is used for the purification of acetylene and artificial textile fibers, and as an antioxidant for rubber (Dequidt et al 1974). It has also been used to extract herbicide residues from soils (Smith 1980), to remove tars and other compounds from petroleum hydrocarbons, and to extract fatty acids from vegetable and fish liver oil. Acetonitrile is now a standard solvent component in reversed-phase high-performance liquid chromatography. It is the starting point for the syntheses of a number of organic compounds such as carboxylic acids and various nitrogen derivatives (Smiley 1981).
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by several routes. An experimental teratogen. Other experimental reproductive effects. A skin and severe eye irritant. Human systemic effects by ingestion: convulsions, nausea or vomiting, and metabolic acidosis. Human respiratory system effects by inhalation. Mutation data reported. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosion Hazard: See also CYANIDE and NITRILES. When heated to decomposition it emits highly toxic fumes of CNand NOx,. Potentially explosive reaction with lanthanide perchlorates and nitrogen-fluorine compounds. Exothermic reaction with sulfuric acid at 53°C. Will react with water, steam, acids to produce toxic and flammable vapors. Incompatible with oleum, chlorosulfonic acid, perchlorates, nitrating agents, inchum, dinitrogen tetraoxide, N-fluoro compounds (e.g., perfluorourea + acetonitrile), HNO3, so3. To fight fire, use foam, Con, dry chemical
Potential Exposure
Acetonitrile is used as an extractant for animal and vegetable oils, as a solvent; particularly in the pharmaceutical industry, and as a chemical intermediate in pesticide manufacture; making batteries and rubber products. It is present in cigarette smoke
Carcinogenicity
Under the conditions of these 2- year inhalation studies by NTP, there was equivocal evidence of carcinogenic activity of acetonitrile in male F344/N rats based on marginally increased incidences of hepatocellular adenoma and carcinoma. There was no evidence of carcinogenic activity of acetonitrile in female F344/N rats exposed to 100, 200, or 400 ppm. There was no evidence of carcinogenic activity of acetonitrile in male or female B6C3F1 mice exposed to 50, 100, or 200 ppm. Exposure to acetonitrile by inhalation resulted in increased incidences of hepatic basophilic foci in male rats and of squamous hyperplasia of the forestomach in male and female mice.
Environmental Fate
Biological. Resting cell suspensions of the soil methylotroph Methylosinus trichosporium OB-
3b rapidly metabolized acetonitrile via oxygen insertion into the C-H bond generating the
intermediate formaldehyde cyanohydrin. The latter compound loses hydrogen cyanide yielding
formaldehyde which is then oxidized to formate (HCO2H) and bicarbonate ion (Castro et al.,
1996).
Photolytic. A rate constant of 4.94 x 10-14 cm3/molecule?sec at 24 °C was reported for the vaporphase
reaction of acetonitrile and OH radicals in air (Harris et al., 1981). Reported rate constants
for the reaction of acetonitrile and OH radicals in the atmosphere and in water are 1.90 x 10-14 and 3.70 x 10-14 cm3/molecule?sec, respectively (Kurylo and Knable, 1984). The estimated lifetime of
acetonitrile in the atmosphere is estimated to range from 6 to 17 months (Arijs and Brasseur,
1986).
Chemical/Physical. The estimated hydrolysis half-life of acetonitrile at 25 °C and pH 7 is
>150,000 yr (Ellington et al., 1988). No measurable hydrolysis was observed at 85 °C at pH
values 3.26 and 6.99. At 66.0 °C (pH 10.42) and 85.5 °C (pH 10.13), the hydrolysis half-lives
based on first-order rate constants were 32.2 and 5.5 d, respectively (Ellington et al., 1987). The
presence of hydroxide or hydronium ions facilitates hydrolysis transforming acetonitrile to the
intermediate acetamide which undergoes hydrolysis forming acetic acid and ammonia (Kollig,
1993). Acetic acid and ammonia formed react quickly forming ammonium acetate.
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 28 mg/L. The adsorbability of the carbon used was 194 mg/g carbon (Guisti et
al., 1974).
Burns with a luminous flame (Windholz et al., 1983), releasing toxic fumes of hydrogen
cyanide.
Metabolism
Acetonitrile metabolism in dogs was demonstrated by Lang (1894), who reported that about 20% of the nitrile administered was converted to thio-cyanate in the urine, while guinea pigs metabolized acetonitrile to a greater extent (50% of dose excreted as thiocyanate). When the animals were pre-treated with ethanol, acetonitrile metabolism was induced (Tanii and Hashimoto 1986). In rats, acetone was found to potentiate acetonitrile toxicity and elevate cyanide concentrations in the blood (Freeman and Hays 1985). Baumann et al (1933) found that rabbits injected with acetonitrile excreted 27-35% of the dose as thiocyanate, while in thyroidectomized rabbits, the excretion decreased significantly (3-5% of the dose). Thiocyanate excretion was increased notably upon feeding dessicated thyroid to these animals. Hunt (1923) found that powdered sheep thyroid protected mice against acetonitrile toxicity. However, the role played by the thyroid in the detoxication of cyanide to thiocyanate is unclear. It has been suggested that the thyroid may have a role in the microsomal cleavage of cyanide from acetonitrile other than its direct effect on sulphation of cyanide to thiocyanate.
storage
Acetonitrile should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Shipping
UN1648 Acetonitrile, Hazard Class: 3; Labels: 3-Flammable liquid
Purification Methods
Commercial acetonitrile is a by-product of the reaction of propylene and ammonia to acrylonitrile. The following procedure that significantly reduces the levels of acrylonitrile, allyl alcohol, acetone and *benzene was used by Kiesel [Anal Chem 52 2230 1988]. Methanol (300mL) is added to 3L of acetonitrile fractionated at high reflux ratio until the boiling temperature rises from 64o to 80o, and the distillate becomes optically clear down to = 240nm. Add sodium hydride (1g) free from paraffin, to the liquid, reflux for 10minutes, and then distil rapidly until about 100mL of residue remains. Immediately pass the distillate through a column of acidic alumina, discarding the first 150mL of percolate. Add 5g of CaH2 and distil the first 50mL at a high reflux ratio. Discard this fraction, and collect the following main fraction. The best way of detecting impurities is by gas chromatography. Usual contaminants in commercial acetonitrile include H2O, acetamide, NH4OAc and NH3. Anhydrous CaSO4 and CaCl2 are inefficient drying agents. Preliminary treatment of acetonitrile with cold, saturated aqueous KOH is undesirable because of base-catalysed hydrolysis and the introduction of water. Drying by shaking with silica gel or Linde 4A molecular sieves removes most of the water in acetonitrile. Subsequent stirring with CaH2 until no further hydrogen is evolved leaves only traces of water and removes acetic acid. The acetonitrile is then fractionally distilled at high reflux, taking precaution to exclude moisture by refluxing over CaH2 [Coetzee Pure Appl Chem 13 429 1966]. Alternatively, 0.5-1% (w/v) P2O5 is often added to the distilling flask to remove most of the remaining water. Excess P2O5 should be avoided because it leads to the formation of an orange polymer. Traces of P2O5 can be removed by distilling from anhydrous K2CO3.
Toxicity evaluation
If released to ambient air, acetonitrile will remain in the vapor phase where it will be degraded through reaction with photochemically produced hydroxyl radicals. The half-life of acetonitrile in ambient air has been estimated to be about 620 days. If released to soil, acetonitrile is expected to volatilize rapidly. Biodegradation in soil is not expected to be a major degradation pathway. If released to water, acetonitrile is not likely to adsorb to soil and sediment particles. Acetonitrile is expected to be removed from water bodies through volatilization, as the chemical hydrolysis and bioaccumulation potential for this chemical are low.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, chlorosulfonic acid, oleum, epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with nitrogen oxide removal from effluent gases by scrubbers or incinerators
Acetonitrile Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Yaokan Chemical Co., LTD | +86-021-60956847 +8613524860067 | ni.chen@yaokanchem.com | China | 3 | 58 |
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 | marketing@royal-chem.com | China | 142 | 55 |
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 | sales@yanshuochem.com | China | 101 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15745 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Nanjing Deda New Material Technology Ltd. | +8613223281135 | niki@njdeda.com | China | 76 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 147 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
Related articles
- Is acetonitrile a highly polar solvent?
- Acetonitrile is a highly polar, volatile organic compound used as a solvent in many industrial applications.
- Dec 27,2023
- Effect of Additives on Acetonitrile Polarity
- Acetonitrile is a relatively nontoxic, highly volatile and aprotic polar organic solvent, some additives can affect its polari....
- Dec 21,2023
- What is Acetonitrile?
- Acetonitrile is a liquid with an etherlike odor. It is a highly polar, volatile solvent used in many different industrial appl....
- Oct 14,2021
View Lastest Price from Acetonitrile manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Acetonitrile
75-05-8
|
US $0.00 / Kg/Drum | 1KG | 99.9% | 5000mt | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-25 | Acetonitrile
75-05-8
|
US $15.00 / kg | 0.1kg | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-25 | Acetonitrile
75-05-8
|
US $1.00 / g | 1g | 99 | 20tons | Shanghai Longyu Biotechnology Co., Ltd. |
-

- Acetonitrile
75-05-8
- US $0.00 / Kg/Drum
- 99.9%
- Jinan Finer Chemical Co., Ltd
-

- Acetonitrile
75-05-8
- US $15.00 / kg
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
-

- Acetonitrile
75-05-8
- US $1.00 / g
- 99
- Shanghai Longyu Biotechnology Co., Ltd.
75-05-8(Acetonitrile )Related Search:
1of4