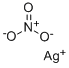Silver nitrate
- CAS No.
- 7761-88-8
- Chemical Name:
- Silver nitrate
- Synonyms
- SILVER(I) NITRATE;Silver nitrate solution;Silbernitrat;Silver Nitrate, Meets analytical specification of Ph. Eur. BP, USP;argerol;Titripur?;BETZ 0207;caswellno737;lunarcaustic;Silver nitrat
- CBNumber:
- CB2280970
- Molecular Formula:
- AgNO3
- Molecular Weight:
- 169.87
- MDL Number:
- MFCD00003414
- MOL File:
- 7761-88-8.mol
- MSDS File:
- SDS
| Melting point | 212 °C (dec.) (lit.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 444°C | ||||||||||||||
| Density | 4.35 g/mL at 25 °C(lit.) | ||||||||||||||
| vapor density | 5.8 (vs air) | ||||||||||||||
| vapor pressure | 17.535 mm of Hg (@ 20°C) | ||||||||||||||
| Flash point | 40 °C | ||||||||||||||
| storage temp. | 2-8°C | ||||||||||||||
| solubility | H2O: soluble | ||||||||||||||
| form | Solid | ||||||||||||||
| color | White | ||||||||||||||
| Odor | Odorless | ||||||||||||||
| PH Range | 7 - 9 | ||||||||||||||
| PH | 5.4-6.4 (100g/l, H2O, 20℃) | ||||||||||||||
| Evaporation Rate | >1 | ||||||||||||||
| Water Solubility | 219 g/100 mL (20 ºC) | ||||||||||||||
| Sensitive | Light Sensitive | ||||||||||||||
| Merck | 14,8518 | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Space group | Pbca | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits |
ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.01 mg/m3 |
||||||||||||||
| InChIKey | SQGYOTSLMSWVJD-UHFFFAOYSA-N | ||||||||||||||
| CAS DataBase Reference | 7761-88-8(CAS DataBase Reference) | ||||||||||||||
| Indirect Additives used in Food Contact Substances | SILVER NITRATE | ||||||||||||||
| Substances Added to Food (formerly EAFUS) | SILVER NITRATE | ||||||||||||||
| FDA 21 CFR | 176.300; 310.545 | ||||||||||||||
| EWG's Food Scores | 2-3 | ||||||||||||||
| FDA UNII | 95IT3W8JZE | ||||||||||||||
| NCI Drug Dictionary | silver nitrate | ||||||||||||||
| ATC code | D08AL01 | ||||||||||||||
| NIST Chemistry Reference | silver(I) nitrate(7761-88-8) | ||||||||||||||
| EPA Substance Registry System | Silver nitrate (7761-88-8) | ||||||||||||||
| Pesticides Freedom of Information Act (FOIA) | Silver nitrate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS05,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H290-H314-H410 | |||||||||
| Precautionary statements | P210-P260-P273-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C,O,N,Xi | |||||||||
| Risk Statements | 34-50/53-8-36/38-51/53-52/53-35-10-40-20/22-22 | |||||||||
| Safety Statements | 26-45-60-61-36/37/39-27-57-37 | |||||||||
| RIDADR | UN 1493 5.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VW4725000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28432100 | |||||||||
| Toxicity | LD50 oral in rat: 1173mg/kg | |||||||||
| NFPA 704 |
|
Silver nitrate Chemical Properties,Uses,Production
description
Silver nitrate is the most important silver compound and is used extensively for the manufacture of silver halide photosensitive material. Pure silver nitrate crystal is stable to light and is easily to be reduced to black metallic silver in the presence of organic matter. Wet silver nitrate and silver nitrate solution can be easily subject to decomposition upon light. Silver nitrate is a kind of oxidizing agent and can cause protein coagulation with corrosive effect on the human body. It has a relative density of 4.35 (19/4 ℃) and a melting point of 212 ℃. Upon being heated to 444 ℃, it can be decomposed into metallic silver, nitrogen dioxide and oxygen. It can be heated and melt into a light yellow liquid in a porcelain crucible and then condensed into white crystals after being cold. If you further increase the temperature, then it is gradually decomposed and can generate brown oxide vapor at the same time. When electric current passes through the silver nitrate solution, metallic silver is deposited on the negative electrode. Silver nitrate is easily soluble in ammonia and water, slightly soluble in alcohol, insoluble in acetone, benzene, and almost insoluble in concentrated sulfuric acid. Its aqueous solution was acidic (pH = 5~6). Silver nitrate, in the aqueous solution of ammonia, meets glucose and formaldehyde can be reduced to generate "silver mirror." Zinc, cadmium, tin, lead, copper and other metals are easy to replace the metallic silver in the nitrate silver solution. Silver nitrate, when being mixed with sulfur, can lead to explosion upon being beaten by hammer.
Silver nitrate is an inorganic silver salt and can dissociate silver ions with sterilization, corrosion, erosion, and convergence effect. Its dilute solution has antibacterial and astringent effect while its concentrated solution has a corrosive effect. Topical application of silver ion can bind with bacterial protein to form silver protein precipitate with bactericidal effect. It can be used for treating dentin hypersensitivity, mucosal ulceration, early caries and cavity disinfection.

silver nitrate powder
Chemical Properties
It is colorless transparent orthorhombic crystal flake. It is easily soluble in water and ammonia, soluble in ether and glycerin, slightly soluble in ethanol, but almost insoluble in concentrated nitric acid. Its aqueous solution exhibits weak acidity.
Uses
Silver nitrate can be used as the raw material of the silver salt, photographic materials, preservatives, and catalyst and also used for silvering, mirror production, etc.
It can be used for analysis reagents.
It can be used for the manufacture of movie film, X-ray photographic film and other photographic emulsions photosensitive material. In the field of electronics industry, it can be used for the manufacture of conductive adhesives, gas purification agents, as well as silvering of electronic components. It can also be used for the silvering material of mirror production and thermal glass liner, voltage-sharing coat and gloves for electronic work. It can also be used for the silvering of other crafts. Battery industry applied it for the production of silver-zinc battery. In the field of medicine, it can be used for sterilization, corrosive reagent. Daily chemical industry used it for the manufacturing of dyed hair shampoo. It can also be applied to the manufacture of other silver catalyst.
It can be used for the cyanide-free silver-plating such as being the major salt of thiosulfate silvering, hydrochloric acid silvering, imino ammonium di-sulphonate silvering and sulphosalicylic acid silvering. It is also the source of the silver ion. The content of the silver nitrate has certain effect on the conductivity, dispersion property and sedimentation speed of the silver-plating solution. The general usage amount is about 25~50 g/L.
Reactions
Silver nitrate can have precipitation reaction and coordination reaction with a series of reagents.
It can react with hydrogen sulfide to form a black silver sulfide Ag2S precipitate.
It can react with potassium chromate, to form a red-brown silver chromate Ag2CrO4 precipitate.
It can react with disodium hydrogen phosphate to form a yellow silver phosphate Ag3PO4 precipitate.
It can react with halogen ion to form silver halide AgX precipitate.
It can react with alkali to form a brown-black silver oxide Ag2O precipitate.
It can react with NH3, CN-, SCN-, S2O3, etc., to form a variety of complex ions, such as: Ag (NH3) 2OH, Ag [Ag (CN) 2], [Ag (SCN) 2]-, [Ag (S2O3) 2] 3-and so on.
It can react with oxalate ions to form white oxalate ion Ag2C2O4 precipitate.
Silver nitrate is a moderately strong oxidant that can be reduced by a number of moderately strong or strong reducing agent to become elemental silver.
Hydrazine (N2H4) and phosphorous acid and reduce Ag+ to metallic silver with the reaction equation: (1) N2H4 + 4AgNO3─ → 4Ag + N2 + 4HNO3; (2) H3PO3 + 2AgNO3 + H2O─ → 2Ag + H3PO4 + 2HNO3
The above information is edited by the chemicalbook of Dai Xiongfeng.
Synthesis
Synthesis method: put the silver bar into the reactor, add distilled water first, followed by adding concentrated nitric acid to make the concentration of nitric acid be about 60% to 65%. Control the heating rate to make sure that the reaction was not too fierce. Heat to above 100 ℃, maintain the vapor pressure at 0.2 MPa and have the reaction for 2~3 h. Release the nitric oxide gas. The material liquid was pumped to a storage tank, diluted with distilled water to a relative density of 1.6 to 1.7. Cool and stand for 10 h and filter to remove impurities such as AgCl. Send the supernatant into an evaporator for being evaporated under reduced pressure at about pH = 1. Cool, crystallize and apply vacuum drying to obtain the products.
Ag + 2HNO3 → AgNO3 + H2O + NO2 ↑
Toxicity
It is corrosive to the skin and mucous membranes and has convergence effect. Skin, upon contact with silver nitrate, will turn dark upon light and is prone to get inflammation. If the skin is contaminated by silver nitrate, you can use iodine remove graze; if skin get injured upon contact, you can soak in salt water for washing.
Upon working, the production staff should wear masks, cotton overalls, and latex gloves and other protective equipment. The production staff should also do laundry frequently. The production equipment should be sealed with the workshop being ventilated.
Silver nitrate can react with acetylene to generate silver acetylene. Under dry conditions, it will explode upon a slight friction. Therefore, upon equipment maintenance, we should prohibit to bring calcium carbide paste and acetylene gas into the workshop.
Chemical Properties
Silver nitrate, AgN03, is colorless,transparent,tabular,rhombic crystals that become gray or grayish-black on exposure to light in the presence of organic matter.It is odorless with a bitter,caustic,metallic taste. It is caustic,and a strong oxidizing agent that is soluble in cold water, more soluble in hot water, glycerol,and hot alcohol,slightly soluble in ether,and decomposes at boiling point Used in photographic film, silver plating,silvering mirrors,and as an antiseptic.
Chemical Properties
Silver nitrate is a colorless to dark gray, odorless, crystalline solid.
Physical properties
Colorless, transparent, large rhombohedral crystals, or white small crystals; bitter, caustic metallic taste; odorless; pure compound is not sensitive to light but trace organics promote photo reduction, turning the salt to grayish black on exposure to light; density 4.35 g/cm3; melts at 212°C; decomposes at 440°C; very soluble in water, soluble in ethanol and acetone.
Uses
The basis of nearly all photographic silver halides with the exception of the daguerreotype process, silver nitrate is a heavy white crystal made by dissolving elemental silver in nitric acid followed by evaporation. It is soluble in water, ether, and glycerin. Silver nitrate is not sensitive to light, but when combined with an organic material, a halogen, or a halide it will reduce back to a metallic state when exposed to light.
Uses
Photographic emulsions, antiseptic, silver plating, and inks.
Uses
Anti-infective, topical.
Indications
Silver nitrate, 0.1% to 0.5%, is an excellent germicide and astringent. Its
germicidal action is due to precipitation of bacterial protein by liberated silver
ions. It may cause pain if applied in concentrations >0.5%.
Silver nitrate is another cauterizing agent and coagulates cellular protein and
removes granulation tissue. This should be applied everyday for approximately
5 days."
"Silver nitrate (AgNO3), in solid form or in solutions stronger than 5%, is used for
its caustic action; 5% to 10% solutions may be applied to fissures or excessive
granulation tissue. Silver nitrate sticks consist of a head of toughened silver nitrate (>94.5%) prepared by fusing the silver salt with sodium chloride. They
are dipped in water and applied as needed.
Preparation
Silver nitrate is prepared by dissolving silver metal in dilute nitric acid. The solution is evaporated and residue is heated to dull red heat with concentrated nitric acid to decompose impurities such as copper nitrate. Residue then is dissolved in water, filtered, and recrystallized to obtain pure silver nitrate.
Definition
ChEBI: Silver(1+) nitrate is a silver salt and an inorganic nitrate salt. It has a role as an astringent.
General Description
A colorless or white crystalline solid becoming black on exposure to light or organic material.
Air & Water Reactions
Water soluble.
Reactivity Profile
Silver nitrate is noncombustible but, as an oxidizing agent, can accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires. Light sensitive. Mixtures with alkyl esters may explode owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. Reacts with acetylene in the presence of ammonia to form silver acetylide, a powerful detonator when dry [Bretherick 1979 p. 198]. Reaction with ethyl alcohol (or other alcohols) may produce silver fulminate, which can explode when disturbed [Bretherick 1979 p. 200]. An intimate mixture of Silver nitrate and magnesium may ignite spontaneously on contact with a drop of water [Bretherick 1979 p. 200]. An explosion occurred when purified phosphine was passed rapidly into a concentrated solution of Silver nitrate [Mellor 3:471 1946-47]. When a mixture of 28% ammonium hydroxide and Silver nitrate solution was treated with a small amount of sodium hydroxide. Black precipitate, silver nitride exploded on stirring [MCA Case History 1554 1968].
Hazard
Strong irritant to skin and tissue.
Health Hazard
Concentrated solutions will produce irritation, ulceration, and discoloration of the skin; also causes severe irritation of the eyes. Ingestion will produce violent abdominal pain and other gastroenteric symptoms.
Fire Hazard
Behavior in Fire: Increases flammability of combustibles.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Silver nitrate (AgNO3), after salicylic acid, is widely used for the treatment of warts. AgNO3 is a highly water-soluble salt, which readily precipitates as AgCl, black in colour, when in contact with the skin. Warts are caused by a human papillomavirus, and mostly hands, feet and the anogenital areas are affected. The treatment is based on the destruction of the local tissue, and the silver salt is applied via a caustic pen to the affected area. Silver nitrate is highly corrosive and is known to destroy these types of tissue growth. Care has to be taken when this treatment option is used, as the resulting AgCl stains any skin or fabric which it has been in contact with.
Safety Profile
A human poison. Experimental poison by ingestion, intravenous, subcutaneous, and intraperitoneal routes. Experimental reproductive effects. Human mutation data reported. A severe eye irritant. A powerful caustic and irritant to skin, eyes, and mucous membranes. Swallowing can cause severe gastroenteritis that may be fatal. Questionable carcinogen with experimental tumorigenic data. A powerful oxidizer. Incompatible with acetylene, acetylides, alkalies, aluminum, antimony salts, arsenic, arsenites, bromides, carbon, carbonates, chlorides, ClF3, chlorosulfuric acid, copper, creosote, ethanol, ferrous salts, hypophosphites, iodides, Mg powder with H20, morphme salts, NH3 with KOH to yield black Ag3N, oils, PH3, phosphates, phosphonium iodide, phosphorus, plastics, sulfur, tannic acid, tartrates, thiocyanates, vegetable decoctions and extracts, zinc with NH3 with KOH. When heated to decomposition it emits toxic fumes of NOx. See also SILVER COMPOUNDS and NITRATES
Potential Exposure
Silver nitrate is used in photography, silver plating; as an antiseptic; in chemical reactions; and mirror manufacturing; as starting material in production of other silver compounds.
Shipping
UN1493 Silver nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
Purify it by recrystallisation from hot water (solubility of AgNO3 in water is 992g/100mL at 100o and 122g/100mL at 0o). It has also been purified by crystallisation from hot conductivity water by slow addition of freshly distilled EtOH. CAUTION: avoid using EtOH for washing the precipitate; and avoid concentrating the filtrate to obtain further crops of AgNO3 owing to the risk of EXPLOSION (as has been reported to us) caused by the presence of silver fulminate. When using EtOH in the purification, the apparatus should be enveloped in a strong protective shield. [Tully, News Ed (Am Chem Soc) 19 3092 1941; Garin & Henderson J Chem Educ 47 741 1970, Bretherick, Handbook of Reactive Chemical Hazards 4th edn, Butterworths, London, 1985, pp 13-14.] Before being used as a standard in volumetric analysis, analytical reagent grade AgNO3 should be finely powdered, dried at 120o for 2hours, then cooled in a desiccator. Recovery of silver residues as AgNO3 [use protective shield during the whole of this procedure] can be achieved by washing with hot water and adding 16M HNO3 to dissolve the solid. Filter this through glass wool and concentrate the filtrate on a steam bath until precipitation commences. Cool the solution in an ice-bath and filter the precipitated AgNO3. Dry it at 120o for 2hours, then cool it in a desiccator in a vacuum. Store it over P2O5 in a vacuum in the dark. AVOID contact with hands due to formation of black stains.
Incompatibilities
A strong oxidizer. Reacts violently with combustible and reducing materials. Reacts with acetylene forming a shock-sensitive explosive. Reacts with alkalis, antimony salts; ammonia, arsenites, bromides, carbonates, chlorides, iodides, hydrogen peroxide; thiocyanates, ferrous salts; oils, hypophosphites, morphine salts; creosote, phosphates, tannic acid; tartarates, halides, vegetable extracts, and others. Attacks some forms of plastics, rubber, and coatings.
Silver nitrate Preparation Products And Raw materials
Raw materials
Preparation Products
1of5