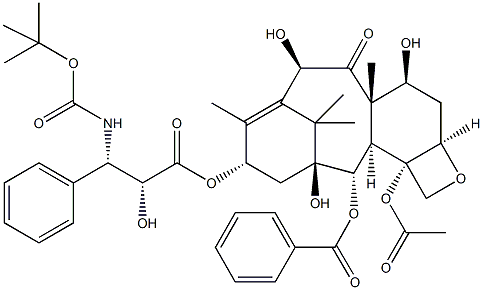
Docetaxel
- CAS No.
- 114977-28-5
- Chemical Name:
- Docetaxel
- Synonyms
- TAXOTERE;rp56976;BIND-014;Doxetaxel;Decetaxel;Anhydrous Docetaxel;Docetere;Docetaxel;Docetaxet;NSC_628503
- CBNumber:
- CB1163212
- Molecular Formula:
- C43H53NO14
- Molecular Weight:
- 807.88
- MDL Number:
- MFCD00871399
- MOL File:
- 114977-28-5.mol
- MSDS File:
- SDS
| Melting point | 186-192 °C (dec.) |
|---|---|
| alpha | -36 º (c=0.74,EtOH) |
| Boiling point | 900.5±65.0 °C(Predicted) |
| Density | 1.38 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Practically insoluble in water, freely soluble in anhydrous ethanol, soluble in methylene chloride. |
| pka | 11.20±0.46(Predicted) |
| color | White |
| Water Solubility | Soluble in dimethyl sulfoxide and ethanol. Insoluble in water. |
| Merck | 14,3397 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChIKey | BEDLLNJKXXVTSX-LWWLJZAUSA-N |
| LogP | 2.456 (est) |
| CAS DataBase Reference | 114977-28-5(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | docetaxel; Taxotere |
| FDA UNII | 699121PHCA |
| NCI Drug Dictionary | docetaxel |
| ATC code | L01CD02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H319-H341-H360D-H362-H372 |
| Precautionary statements | P202-P260-P263-P302+P352-P305+P351+P338-P308+P313 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38-61 |
| Safety Statements | 26-36/37-53-45 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | DA4172750 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29322090 |
| Toxicity | LD50 intravenous in dog: 2500ug/kg |
Docetaxel price More Price(69)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 01885 | Docetaxel purum, ≥97.0% (HPLC) | 114977-28-5 | 5MG-F | $269 | 2024-03-01 | Buy |
| Sigma-Aldrich | 01885 | Docetaxel purum, ≥97.0% (HPLC) | 114977-28-5 | 25MG-F | $926 | 2024-03-01 | Buy |
| Sigma-Aldrich | 01885 | Docetaxel purum, ≥97.0% (HPLC) | 114977-28-5 | 5mg | $259 | 2023-01-07 | Buy |
| Sigma-Aldrich | 01885 | Docetaxel purum, ≥97.0% (HPLC) | 114977-28-5 | 25mg | $890 | 2023-01-07 | Buy |
| Alfa Aesar | J60174 | Docetaxel, 99% | 114977-28-5 | 100mg | $185 | 2024-03-01 | Buy |
Docetaxel Chemical Properties,Uses,Production
Indications and Usage
Docetaxel is a taxane, antineoplastic, and anticancer drug used to treat locally advanced or metastatic breast cancer, even after the failure of cisplatin therapy. Its anti-tumor efficacy has been proven in in vitro tests to be 1.3-12 times that of paclitaxel. Clinical studies have shown that it is more effective than paclitaxel against anthracycline resistant breast cancer. Docetaxel is currently the most effective for the second-line treatment of anthracycline resistant breast cancer. In single cell therapy and combined chemotherapy for non-small cell lung cancer, it is one of the most effective drugs. It can be used for cancer of the uterus, and to study antibiotics, cell biology, cell signaling, neuroscience, apoptosis, and cell cycles. It is also used to study hair loss caused by docetaxel chemotherapy, and to prevent and treat small-cell lung cancer in patients contraindicated for chemotherapy. It is also used to investigate the roles of hypoxia-inducible factors-1α and 2α in androgen-insensitive prostate cancer cells.
Mechanisms of Action
Docetaxel promotes microtubule stabilization by promoting the assembly of microtubule dimers into microtubules, and preventing depolymerization, thus blocking cells in the G2 and M stages, and inhibiting the mitosis and proliferation of cancer cells. Its pharmacological effects are stronger than those of paclitaxel, with 3 times the intracellular concentration and long retention time within the cell, and twice the microtubule affinity; As a microtubule stabilizer and assembly promoter, its activity is twice that of paclitaxel; as a microtubule depolymerization inhibitor, its activity is also twice that of paclitaxel.
Pharmacokinetics
In a pharmacokinetic study in which cancer patients took 20-115 mg/m2, when the dose was 75-115 mg/m2 over a 1-2 hour intravenous drip, AUC was correlated with dose. Its pharmacokinetic characteristics conform to a three-compartmental pharmacokinetic model, with α, β, and γ half-lives of 4, 36, and 11.1 hours respectively. The concentration of the initial phase decreased rapidly, indicating that the drug was distributed to the peripheral compartment. The latter phase is partly due to the relatively slow elimination of the drug from the compartment. Within an hour of intravenous infusion of 100 mg/m2 of paclitaxel, the average peak was 3.7 ug/ml, AUC was 4.6 ug/ml•h, and total clearance and steady-state distribution were 21 L/h/m2 and 113 L. In vitro studies have shown that it has a plasma protein binding rate of more than 94-97%, and dexamethasone does not affect its protein binding. Docetaxel and its metabolites are mainly excreted from feces. Fecal and urinary excretion accounted for 75% and 6% of the dose, and only a small part is excreted in original form. In vitro studies have shown that docetaxel is metabolized by CYP3A4 isoenzymes, which can be inhibited by CYP3A4 inhibitors.
Clinical Research
Genotoxicity: docetaxel induced fractures in CHO-K1 cellular chromosome aberration tests and mouse bone marrow micronucleus tests, but no mutagenicity was observed in Ames tests and CHO/HGPRT gene mutation tests. Reproductive toxicity: no damage to fertility upon intravenous injection 0.3 mg/kg in mice (calculated by body surface area, about 1/50 of the recommended clinical dosage), but could cause reduction in testicular weight. This result is correlated with the results of repeatedly administering it to rats and dogs for 10 cycles (administered once every 21 days, for 6 months); when the intravenous doses were 5 mg/kg and 0.375 mg/kg respectively (converted by surface area, 1/3 and 1/15 of the recommended clinical dose, respectively), testicular atrophy and degeneration were observed. Similar effects were also observed in rats when given low doses more frequently. When rats and rabbits were given ≥0.3 mg/kg/day and 0.03 mg/kg/day (converted from surface area, respectively equivalent to 1/50 and 1/300 of the clinically recommended dose) during organogenesis), embryonic and fetal toxicity was observed (manifesting as intrauterine death, absorbed fetus, and fetal weight loss and delayed ossification). The above dose may case maternal toxicity.
Drug Interactions
In vitro studies have found that CYP3A4 inhibitors may interfere with the metabolism of docetaxel, so it should be used with caution in combination with such drugs (such as ketoconazole, erythromycin, cyclosporine, etc.)
Adverse Reactions
1. Bone marrow suppression: neutropenia is the most common adverse reaction is usually severe (under 500/mm3). Reversible and does not accumulate. Fever and infection associated with neutropenia have been reported in the literature. Anemia can be seen in most cases, with rare severe thrombocytopenia. 2. Allergic reactions: some patients experience severe allergic reactions, characterized by hypotension and bronchospasm, requiring interruption of treatment. The patient can return to normal immediately after discontinuing treatment. Mild allergic reactions, such as blush (with or without itchy erythema), chest tightness, back pain, difficulty breathing, drug fever, or chills, occur in some patients. 3. Skin reactions are usually manifest as erythema, mainly local rashes on hands and feet, sometimes also on arms, face, and chest, sometimes accompanied by itching. Rashes usually occur within a week of infusion, but can return before the next infusion. Severe symptoms, such as peeling after skin rash, occur rarely. Fingernail (or toenail) lesions may occur, characterized by hyperpigmentation or thinning, and sometimes pain and nail loss. 4. Fluid retention: edema, and a few reported cases of pleural effusion, ascites, pericardial effusion, increased capillary permeability, and weight gain. Lower limb fluid retention after four cycles of treatment or cumulative dose of 400mg/m2 may develop into systemic edema, and weight gain of 3kg or more. After discontinuing treatment, fluid retention gradually disappears. Corticosteroids should be given to prophylactically reduce fluid retention. 5. Gastrointestinal reactions such as nausea, vomiting, or diarrhea may occur. 6. There have been reports of neurotoxicity in clinical trials. 7. Cardiovascular adverse reactions such as hypotension, sinus tachycardia, palpitations, pulmonary edema, and hypertension may occur. 8. Other adverse reactions may include: hair loss, weakness, mucositis, joint and muscle pain, hypotension, and reaction at injection site. 9. Patients with normal liver function have also experienced elevated transaminase during treatment. For those with elevated bilirubin, the relationship with docetaxel is still unclear.
Contradictions
1 Patients with a history of severe allergic sensitivity to docetaxel or polysorbate-80.
2 Patients with white blood cell count under 1500/mm3.
3 Patients with severe liver damage.
Precautions
At present there is still insufficient strictly controlled clinical data for pregnant women. Patient who are a pregnant woman or become pregnant while using docetaxel should be informed of potential damage to the fetus and risk of miscarriage. Women who may become pregnant during treatment should use birth control. It is not clear whether docetaxel is excreted from human milk. Given that many drugs may be excreted from human milk, and that docetaxel may have serious adverse reactions for breastfeeding infants, mothers should stop breastfeeding before treatment.
The efficacy and safety of docetaxel in children has not yet been determined.
Description
Docetaxel, a semi-synthetic product from the taxoid family, was launched in 1995 first in South Africa and subsequently in several other markets for the treatment of ovarian, breast and non-small cell lung cancers. Like the naturally occurring antitumor agent paclitaxel, the first marketed taxoid, docetaxel promotes both the rate and extent of tubulin assembly into stable microtubules and inhibits their depolymerization. It acts as a mitotic spindle poison and induces a mitotic block in proliferating cells. This mechanism of action for taxoids is unique from other classes of anticancer agents. Docetaxel was reported to be twice as potent as paclitaxel in several in vitro protocols and also exhibit higher cytotoxicity. Clinical trials are on going for docetaxel for other types of tumors including pancreatic, gastric, head and neck cancers and soft tissue sarcomas.
Chemical Properties
Off-white Cryst
Originator
Rhone-Poulenc Rorer (France)
Uses
antineoplastic;binds to microtubules
Uses
Docetaxel used to treat variety of cancers (Lung, Breast, Prostate). It is a second-generation cytotoxic antimicrotubule agent and is mostly used in the pharmaceutical industry.
Uses
Docetaxel is a semisynthetic analog of taxol that inhibits microtubule disassembly (IC50 = 0.2 μM) and inhibits cell replication (IC50 = 0.13 μM). It has proven more effective than taxol in preventing the proliferation of cancer cells. Docetaxel has applications in breast cancer and hormone-refractory prostate cancer. This product is intended for research applications.
Definition
ChEBI: A tetracyclic diterpenoid that is paclitaxel with the N-benzyloxycarbonyl group replaced by N-tert-butoxycarbonyl, and the acetoxy group at position 10 replaced by a hydroxy group.
Manufacturing Process
Taxol, a material occurring in nature, and extracted from Taxus brevifolia (i.e.
the Pacific yew tree). It consists of the A, B and C variants. Taxol is not water
soluble, thereby complicating its delivery in vivo for therapeutic purposes.
A sample of Taxol (14.7 g, 17 mmol) was dissolved in pyridine (150 mL) and
chlorotriethylsilane (23.03 g, 147 mmol) was added. The reaction was stirred
at 25°C under N2. After 20 hours the reaction appeared complete by TLC
analysis (7% MeOH/CH2Cl2). The mixture was concentrated to remove the
pyridine. The residue was dissolved in CH2Cl2 and washed with water, 10%
CuSO4, NaHCO3 and brine successively. The organic layer was dried over
MgSO4, and concentrated to yield 20.89 g of the crude 2,7'-bis(triethylsilyl)
Taxol. A portion of crude 2',7-bis(triethylsilyl) Taxol (14.50 g, 13.4 mmol) was
dissolved in dry THF (150 mL). Zirconocene chloride hydride (7.75 g, 30.2
mmol) was added. The reaction was stirred at 25°C under N2. After 20 hours
the reaction appeared complete by TLC analysis. The mixture was poured intocold hexanes, and the resulting precipitated Zr complexes were filtered off.
The solution was concentrated to yield 17 g of the crude 2,7'-bis(triethylsilyl)
Taxol imine. A portion of crude 2',7-bis(triethylsilyl) Taxol imine (8.36 g) was
dissolved in 1% HCl/EtOH (180 mL) and the reaction was stirred at 25°C for
20 hours. The reaction appeared complete by TLC analysis. The mixture was
poured into 800 mL of water and washed with hexane (180 mL times 3). The
aqueous layer was neutralized with NaHCO3 to pH=7.0. The product was
extracted with CH2Cl2. The organic layer was removed and concentrated to a
solid. Silica gel chromatography (5% MeOH/CH2Cl2) yielded Taxol primary
amine (2.41 g, 52% overall yield based on 5 g of Taxol used). Melting point
160°C-162°C.
A sample of Taxol primary amine (100 mg, 0.13 mmol) was dissolved in
CH2Cl2 (10 mL) and HCl (15 mM in Et2O; 10 ml, 150 mmol) was added. The
reaction was stirred at 25°C for 2 minutes. The mixture was concentrated to
remove the solvents. The residue was redissolved in CH2Cl2 and precipitated
in hexane. Filtration yielded 85 mg of Taxol PA (PA-primary amine) HCl
(83%). Melting point 65°C. A sample of Taxol PA HCl (50 mg, 0.064 mmol)
was dissolved in 0.5 ml of water. It was neutralized to pH 7.0 by addition of
saturated NaHCO3, followed by extraction with CH2Cl2. The organic layer was
concentrated and chromatographed (3% MeOH/CH2Cl2was used as mobile
phase) to yield 30 mg of Taxol primary amine (63% yield). The 1H NMR and
LRMS data agree well with a standard sample of Taxol primary amine.
Trimethylsilyl- and trichlorethoxycarbonyl-protecting group can be used. A
mixture of impure Taxol A, B, C can be converted Taxol primary amine, which
then can be converted to Taxol A or docetaxel.
brand name
Taxotere (Sanofi Aventis).
Therapeutic Function
Antitumor
Hazard
A poison. Human systemic effects.
Clinical Use
Antineoplastic agent:
Treatment of breast cancer, prostate cancer and
non-small cell lung cancer unresponsive to alternative
therapies, also gastric adenocarcinoma, squamous cell
carcinoma of head and neck
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
clarithromycin - avoid or reduce docetaxel dose
Antifungals: concentration possibly increased by
itraconazole and voriconazole - avoid or reduce
docetaxel dose.
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Antivirals: concentration possibly increased by
indinavir, ritonavir and saquinavir avoid or reduce
docetaxel dose.
Ciclosporin: possibly inhibits metabolism of
ciclosporin; bioavailability of docetaxel increased by
ciclosporin.
Metabolism
A study of [14C]-docetaxel has been conducted in three cancer patients. Docetaxel was eliminated in both the urine and faeces following cytochrome P450 3A4-mediated oxidative metabolism of the tert-butyl ester group, within seven days, the urinary and faecal excretion accounted for about 6% and 75% of the administered radioactivity, respectively. About 80% of the radioactivity recovered in faeces is excreted during the first 48 hours as one major inactive metabolite and 3 minor inactive metabolites and very low amounts of unchanged medicinal product.
storage
Store at +4°C
References
1) Fabbri et al. (2008), Mitotic catastrophe and apoptosis induced by docetaxel in hormone-refractory prostate cancer cells; J. Cell Physiol, 217 494 2) Dosso and Berthold (2008), Docetaxel in the management of prostate cancer: current standard of care and future directions; Expert Opin. Pharmacother, 9 1969 3) Homma et al. (2008), RPN2 gene confers docetaxel resistance in breast cancer; Nat. Med., 14 939 4) Kars et al. (2008), Reversal of Multidrug Resistance by Synthetic and Natural Compounds in Drug-Resistant MCF-7 Cell Lines; Chemotherapy, 54 194 5) Wallin et al. (2012), GDC-0941, A Novel Class I Selective PI3K Inhibitor, Enhances the Efficacy of Docetaxel in Human Breast Cancer Models by Increasing Cell Death In vitro and In vivo; Clin Cancer Res.,?18 3901 6) Heinemann et al. (2011), Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer; BMC Cancer, 11 221
Docetaxel Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Mesochem Technology Co.,Ltd | +8613651027935 | rachel@mesochem.com | China | 191 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 47 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 893 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
Related articles
- Docetaxel: activity, mechanism of action and pharmacokinetics
- Docetaxel is a chemotherapy drug that has potent anti-cancer activity against various tumors and has promising effects in prom....
- Jun 7,2023
- Uses and Possible Side Effects of Docetaxel
- Docetaxel works by disrupting the microtubular network in cells, which is essential for cell division and other normal cellula....
- Oct 12,2019
View Lastest Price from Docetaxel manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Docetaxel
114977-28-5
|
US $43.00-35.00 / Grams | 1Grams | 99.99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-25 | Docetaxel
114977-28-5
|
US $0.00-0.00 / Gram | 10Gram | EP | 2kg | Hangzhou Hyper Chemicals Limited | |
 |
2024-04-23 | Docetaxel
114977-28-5
|
US $1.00 / g | 1g | 99% | 10 tons | ANHUI WITOP BIOTECH CO., LTD |
114977-28-5(Docetaxel)Related Search:
1of4