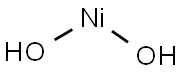
NICKEL(II) HYDROXIDE
- CAS No.
- 12054-48-7
- Chemical Name:
- NICKEL(II) HYDROXIDE
- Synonyms
- Ni(OH)2;nickel(ii);Dihydroxynickel;NICKEL HYDROXIDE;Nickeldihydroxid;nickeldihydroxide;Nickeloushydroxide;Dihydroxynickel(II);Niekelous hydroxide;Nickel(Ⅱ) Hydroxide
- CBNumber:
- CB2411181
- Molecular Formula:
- H2NiO2
- Molecular Weight:
- 92.71
- MDL Number:
- MFCD00011140
- MOL File:
- 12054-48-7.mol
| Melting point | 230°C (dec.) |
|---|---|
| Density | 4.1g/mL(lit.) |
| form | Powder |
| color | Green |
| PH | 8.37(1 mM solution);8.37(10 mM solution);8.37(100 mM solution) |
| Water Solubility | Insoluble in water. Soluble in dilute acid. |
| Merck | 13,6534 |
| Solubility Product Constant (Ksp) | pKsp: 15.26 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability | Stable. Incompatible with strong acids, water, moisture. |
| InChI | InChI=1S/Ni.2H2O/h;2*1H2/q+2;;/p-2 |
| InChIKey | BFDHFSHZJLFAMC-UHFFFAOYSA-L |
| SMILES | [Ni](O)O |
| CAS DataBase Reference | 12054-48-7(CAS DataBase Reference) |
| EWG's Food Scores | 6 |
| FDA UNII | L8UW92NW6J |
| Proposition 65 List | Nickel Hydroxide |
| EPA Substance Registry System | Nickel hydroxide (Ni(OH)2) (12054-48-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H332-H315-H317-H334-H341-H350i-H360D-H372-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P301+P312-P302+P352-P308+P313 | |||||||||
| Hazard Codes | Xn,N,T | |||||||||
| Risk Statements | 20/22-40-43-50/53-68-48/23-42/43-38-61-49 | |||||||||
| Safety Statements | 22-36-60-61-45-53 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QR7040000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28254000 | |||||||||
| NFPA 704 |
|
NICKEL(II) HYDROXIDE price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 283622 | Nickel(II) hydroxide | 12054-48-7 | 250g | $86.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 283622 | Nickel(II) hydroxide | 12054-48-7 | 1kg | $245 | 2024-03-01 | Buy |
| Alfa Aesar | 012517 | Nickel(II) hydroxide, Ni 61% | 12054-48-7 | 250g | $114 | 2024-03-01 | Buy |
| Alfa Aesar | 012517 | Nickel(II) hydroxide, Ni 61% | 12054-48-7 | 1kg | $345 | 2023-06-20 | Buy |
| Strem Chemicals | 93-2847 | Nickel(II) hydroxide | 12054-48-7 | 250g | $71 | 2024-03-01 | Buy |
NICKEL(II) HYDROXIDE Chemical Properties,Uses,Production
Physical Properties
Green hexagonal crystal; density 4.10 g/cm3; decomposes to NiO on heating at 230°C; insoluble in water; KSP 5.47x10-16; monohydrate is insoluble in water but soluble in dilute acids and ammonia.
Preparation
Nickel hydroxide is prepared by various methods, mostly involving reaction of caustic soda or caustic potash with a soluble nickel salt. Thus, treating nickel sulfate solution with sodium hydroxide forms a voluminous green gel. The gel crystallizes on prolonged storage. Alternatively, the solution on neutralization forms a fine precipitate of nickel hydroxide. Nickel nitrate also is used as starting material to prepare nickel hydroxide. Its aqueous solution, on treatment with sodium or potassium hydroxide, yields a gelatinous precipitate of nickel hydroxide which may be extracted with hot alcohol to form high purity product.
Nickel hydroxide in high purity is prepared by an electrolytic process using metallic nickel as the anode and nickel nitrate solution as the electrolyte. Nickel hydroxide is electrodeposited at an inert cathode.
Chemical Properties
Black powder.
Chemical Properties
Nickel hydroxide is a light, apple-green powder.
Uses
Nickel salts.
Uses
Nickel hydroxide was used as a reference material in a study where the influence of stacking faults on the electrochemical activity of Ni(OH)2 electrodes was studied.
Uses
Due to its redox behavior, it finds use in rechargeable battery electrodes. It is useful for the storage of electrochemical energy, with potential applications in car batteries. It is a potential material for several biological applications, for example in chromosomal DNA quantification assay.
Preparation
This is precipitated as finely divided green powder when an alkali metal hydroxide solution is added to an aqueous solution of a nickel(II) salt. It is frequently difficult to filter at first but becomes more crystalline upon prolonged standing. If the nickel salt is incompletely precipitated, especially if very strong solutions of nickel(II) are used, then the precipitate may be a basic salt. This is particularly true of the halides, in which case compounds such as NiCl2?3Ni(OH)2 and NiCl2?Ni(OH)2 have been characterized.
Production Methods
Nickel hydroxide is obtained either by treating nickel sulfate solution with sodium hydroxide or by hot alcohol extraction of the precipitate formed as a result of the reaction of nickel nitrate with potassium hydroxide. Nickel hydroxide is used for the manufacture of nickel–cadmium electric cells, and as an intermediate product during the manufacture of nickel catalysts.
Hazard
Confirmed carcinogen.
Flammability and Explosibility
Non flammable
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by subcutaneous route. See also NICKEL COMPOUNDS.
Potential Exposure
It may be found in the workplace as a dust, liquid, or acid solution. This compound may be used in nickel plating operations.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Incompatible with strong acids. Aqueous solution may be acidic.
Waste Disposal
Recover and recycle where possible or dispose of in a chemical waste landfill.
NICKEL(II) HYDROXIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
View Lastest Price from NICKEL(II) HYDROXIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-13 | Nickel(ii) hydroxide
12054-48-7
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 20 tons | airuikechemical co., ltd. | |
 |
2019-07-06 | NICKEL(II) HYDROXIDE
12054-48-7
|
US $7.00 / kg | 1kg | 99% | 100kg | Career Henan Chemical Co |
-

- Nickel(ii) hydroxide
12054-48-7
- US $0.00-0.00 / Kg
- 99.9%
- airuikechemical co., ltd.
-

- NICKEL(II) HYDROXIDE
12054-48-7
- US $7.00 / kg
- 99%
- Career Henan Chemical Co
12054-48-7(NICKEL(II) HYDROXIDE)Related Search:
1of4