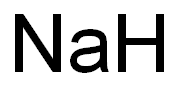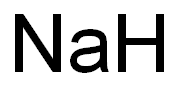Sodium
- CAS No.
- 7440-23-5
- Chemical Name:
- Sodium
- Synonyms
- SODIUM METAL;Natrium;Metallic sodium;Sodium dispersion;Sodium solution;NATRIUMMETALL;SODIUM STANDARD;SODIUM STANDARD SOLUTION;Sodium, Oil based standard solution, Specpure(R), Na 5000μg/g;SODIUM, REAGENT (ACS)SODIUM, REAGENT (ACS)SODIUM, REAGENT (ACS)
- CBNumber:
- CB4854184
- Molecular Formula:
- Na
Lewis structure

- Molecular Weight:
- 22.99
- MDL Number:
- MFCD00085307
- MOL File:
- 7440-23-5.mol
- MSDS File:
- SDS
| Melting point | 97.8 °C (lit.) |
|---|---|
| Boiling point | 883 °C (lit.) |
| Density | 1.04 g/mL at 20 °C |
| vapor pressure | 1 mm Hg ( 440 °C) |
| Flash point | 128 °F |
| storage temp. | water-free area |
| solubility | H2O: soluble |
| form | pieces (large) |
| Specific Gravity | 0.97 |
| color | White to off-white |
| Resistivity | 4.69 μΩ-cm, 20°C |
| Water Solubility | REACTS |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,8570 |
| Exposure limits |
ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability | Reacts violently with water, liberating and possibly igniting hydrogen. Flammable solid. Incompatible with water, strong oxidizing agents. Do not store near oxidants. Store under oil, or dry inert gas. Air sensitive. |
| InChIKey | MPMYQQHEHYDOCL-UHFFFAOYSA-N |
| CAS DataBase Reference | 7440-23-5(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | SODIUM |
| FDA 21 CFR | 101.9; 107.10; 107.100; 175.105; 176.170; 176.200; 178.3910; 310.545 |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | sodium |
| FDA UNII | 9NEZ333N27 |
| NIST Chemistry Reference | Sodium(7440-23-5) |
| EPA Substance Registry System | Sodium (7440-23-5) |
| Hardness, Vickers | 0.10 |
|---|
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS02,GHS05,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H260-H304-H314-H336-H411 | |||||||||
| Precautionary statements | P231+P232-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,F,T | |||||||||
| Risk Statements | 34-14/15-45-65 | |||||||||
| Safety Statements | 26-8-6A-45-43D-43-36/37/39-22-7/8-62-5 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | VY0686000 | |||||||||
| Autoignition Temperature | >115 °C in air | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2805 11 00 | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| NFPA 704 |
|
Sodium Chemical Properties,Uses,Production
Description
Sodium was first isolated by Sir Humphry Davy in 1807 by electrolysis of caustic soda. In the following year, Gay Lussac and Thenard obtained metallic sodium by chemical reduction of caustic soda with iron at elevated temperatures. Deville, in 1854, prepared the metal by reduction of sodium carbonate and lime with charcoal at a temperature above the boiling point of sodium. Castner, in 1886, improved the chemical reduction process preparing the metal by heating sodium hydroxide with iron carbide at high temperature. Five years later he patented a process based on electrolytic reduction of sodium hydroxide. The first major commercial plant was set up in 1921 with the introduction of Downs cell.
The element derived its name from the Latin word sodanum meaning “headache remedy.” Its symbol Na was derived from the Latin word, natrium.

Sodium is the sixth most abundant element on earth. It comprises about 2.6% weight of the earth’s crust. Its salt, sodium chloride, is the major component of seawater. The concentration of sodium in seawater is 1.08%. As a very reactive element, sodium is never found in free elemental form. It occurs in nature in many minerals such as cryolite, amphibole, zeolite, sodalite, and soda niter. Sodium chloride (NaCl) is the most common salt of sodium. Some other important salts are caustic soda (NaOH), soda ash (Na2CO3), baking soda (NaHCO3), Chile saltpeter (NaNO3), borax (Na2B4O7•10H2O), sodium thiosulfate (Na2S2O3), sodium sulfate (Na2SO4), and sodium phosphates.
Uses
Metallic sodium is a strong reducing agent, used in many organic syntheses. It is used in the manufacture of sodamide, sodium peroxide, and esters. Other uses are in purifying molten metals, to descale metal, to improve structure of certain alloys, and as a heat transfer agent, for example, in nuclear reactors. Sodium is useful in producing other metals, such as titanium. It is used in sodium vapor lamps in small amounts. Sodium wire is used to remove traces of water from organic solvents.
Production Methods
Sodium metal is produced by both electrolytic and chemical reduction processes. All commercial processes employed today are based on electrolytic methods. Such processes are in wide use since Davy prepared the metal the first time in 1807.
There are two electrolytic methods that are of major importance. One involves the electrolysis of fused sodium chloride using the Downs cell. This method currently is most prevalent. The Downs cell consists of a steel cell with brick lining containing the fused bath. The multiple electrode arrangement consists of four cylindrical graphite anodes that project upward from the base of the cell. Each anode is surrounded by a diaphragm of iron gauge and a steel cathode.
Fused sodium chloride is electrolyzed at bath temperature varying between 565 to 600°C at a cell voltage of 5.7 to 7 V and the cell current varying from 25 to 35 kA. The cathode current density is mostly about 9.8 kA/m2. Often calcium chloride is added to sodium chloride in the cell bath to lower its melting point. Calcium is largely removed from sodium by filtration at about 110°C. Other electrolyte compositions have been used in which calcium is partially or fully replaced. The cell feed must be free of sulfate and other impurities.
Electrolysis of fused sodium hydroxide has been achieved successfully with a Castner cell. The Castner cell was used in commercial production prior to introduction of Downs cell. The cell is operated at a bath temperature 320 ± 10°C, at 9.0 ± 0.5 amp current and a voltage of 4.3 to 5.0 V. The cathode current density is about 10.9 kA/m2. The cell consists of a copper cathode and a nickel anode and a cylindrical iron-gauge diaphragm placed between the electrodes. The cell reactions are as follows:
cathode: 4Na+ + 4e¯ → 4Na
SODIUM 847anode: 4OH¯ – 4e¯ → 2H2O + O2
Water generated at the anode diffuses through the diaphragm and goes to the cathode, reacting with sodium to form sodium hydroxide.
2H2O + 2Na → 2NaOH + H2
The overall change may be represented as:
2NaOH → 2Na + H2 + O2
Because water is reacting with sodium produced at the cathode, the yield of sodium is reduced almost by 50%. Lesser yield is the major disadvantage of the Castner process. At present, this process is not used commercially.
Thermal reduction processes are not being practiced anywhere in the world at present for large-scale production of sodium. Such methods, however, can be conveniently adapted for laboratory preparation of metallic sodium. Sodium can be prepared by thermal reduction of its hydroxide, carbonate, or chloride at elevated temperatures. These salts are heated with carbon, calcium carbide, iron carbide, ferrosilicon, or other reducing agents at temperatures above 800°C under vacuum:
6NaOH + 2C → 2Na + 2Na2CO3 + 3H2
Na2CO3 + 2C → 2Na + 3CO
2NaCl + CaC2 → 2Na + CaCl2 + 2C
Description
In its ionic form, sodium is one of the most important biological
nutrients and is found nearly everywhere on Earth.
Although it was isolated as a free metal in 1807 by Sir
Humphry Davy and makes up 2.83% of Earth’s lithosphere, it
is not found in its metallic form in nature. Pure sodium is
extremely reactive, particularly with water to form explosive
hydrogen gas and lye (NaOH); it can also react with water
vapor in air or biological tissues.
Mined and refined salts from terrestrial and aquatic sources
contain sodium in the form of sodium chloride, sodium
iodide, and other compounds. Natron, a naturally occurring
mixture of sodium compounds, has been used since the time of
the ancient Egyptians, and sodium compounds are essential to
numerous industries, including those involving glass, paper,
and soap production. Since it does not occur in its metallic
form in nature, pure sodium metal must be produced industrially,
which is accomplished via electrolysis of molten sodium
chloride.
Chemical Properties
Sodium is a soft silvery white metallic element. Pyrophoric solid or molten liquid. Odorless, oxidizing rapidly in air; waxlike at room temperature, brittle at low temperatures. Store in airtight containers or in naphtha or similar liquid that does not contain water or free oxygen. Decomposes water on contact, with evolution of hydrogen to form sodium hydroxide; insoluble in benzene, kerosene, and naphtha. Has excellent elec- trical conductivity and high heat-absorbing capacity.
Physical properties
Sodium is a soft, wax-like silver metal that oxidizes in air. Its density is 0.9674 g/cm3, andtherefore it floats on water as it reacts with the water releasing hydrogen. It has a rather lowmelting point (97.6°C) and a boiling point of 883°C. Sodium is an excellent conductor ofheat and electricity. It looks much like aluminum but is much softer and can be cut with aknife like butter. Its oxidation state is +1.
Isotopes
Sodium has 14 isotopes. The only stable isotope of sodium has an averageatomic weight of 23 (23Na) and makes up about 100% of all the isotopes of the element sodium found on Earth. All the other 13 isotopes (from 19Na to 31Na) are radioactive with relatively short half-lives and thus are unstable.
Origin of Name
The Latin name for the symbol for “sodium” (Na) is natrium, and the name “sodium” in Latin is sodanum, which was known as an ancient headache remedy and was called “soda” in English.
Occurrence
Sodium is the sixth most abundant of the Earth’s elements. Since it is a highly electropositive metal and so reactive with nonmetals, it is not found in its pure elemental form on Earth.Rather, it is found in numerous compounds in relatively abundant quantities. About 2.83%of the Earth’s crust consists of sodium in compounds.Sodium is produced by an electrolytic process, similar to the other alkali earth metals. (Seefigure 4.1). The difference is the electrolyte, which is molten sodium chloride (NaCl, common table salt). A high temperature is required to melt the salt, allowing the sodium cationsto collect at the cathode as liquid metallic sodium, while the chlorine anions are liberated aschlorine gas at the anode: 2NaCl (salt) + electrolysis → Cl2↑ (gas) + 2Na (sodium metal). Thecommercial electrolytic process is referred to as a Downs cell, and at temperatures over 800°C,the liquid sodium metal is drained off as it is produced at the cathode. After chlorine, sodiumis the most abundant element found in solution in seawater.
Characteristics
On the periodic table sodium is located between lithium and potassium. A fresh cut intosodium looks silvery but turns gray as sodium oxidizes rapidly in air, forming sodium oxideon its surface.Sodium is extremely reactive. It reacts explosively in water as it releases hydrogen fromthe water with enough heat to ignite the hydrogen. The resulting compound of this reactionis sodium hydroxide (2Na + 2H2O → 2NaOH + H2↑). Due to its extremely electropositivereactivity, there are few uses for the pure metallic form of sodium. Because of its reactivity,hundreds of sodium compounds are found on the Earth’s surface.Guide to the Elements | 51An unusual characteristic of several alkali metals is that a mixture of two or more has alower melting point than the melting point of the separate metals. This is referred to as aeutectic system of metallic alloys. For instance, sodium has a melting point of 97.6°C, andpotassium’s melting point is 63.25°C, but when the two are mixed, the eutectic melting point(turning into a liquid phase) of the combined Na-K system is below zero degrees Celsius(–10°C). If cesium metal (melting point of 38.89°C) is added to the Na and K mixture, themelting point of this eutectic alloy (Na-K-Cs) is the lowest of any eutectic alloy at –78°C.
History
Long recognized in compounds, sodium was first isolated by Davy in 1807 by electrolysis of caustic soda. Sodium is present in fair abundance in the sun and stars. The D lines of sodium are among the most prominent in the solar spectrum. Sodium is the sixth most abundant element on earth, comprising about 2.6% of the Earth’s crust; it is the most abundant of the alkali group of metals of which it is a member. The most common compound is sodium chloride, but it occurs in many other minerals, such as soda niter, cryolite, amphibole, zeolite, sodalite, etc. It is a very reactive element and is never found free in nature. It is now obtained commercially by the electrolysis of absolutely dry fused sodium chloride. This method is much cheaper than that of electrolyzing sodium hydroxide, as was used several years ago. Sodium is a soft, bright, silvery metal that floats on water, decomposing it with the evolution of hydrogen and the formation of the hydroxide. It may or may not ignite spontaneously on water, depending on the amount of oxide and metal exposed to the water. It normally does not ignite in air at temperatures below 115°C. Sodium should be handled with respect, as it can be dangerous when improperly handled. Metallic sodium is vital in the manufacture of sodamide and esters, and in the preparation of organic compounds. The metal may be used to improve the structure of certain alloys, to descale metal, to purify molten metals, and as a heat transfer agent. An alloy of sodium with potassium, NaK, is also an important heat transfer agent. Sodium compounds are important to the paper, glass, soap, textile, petroleum, chemical, and metal industries. Soap is generally a sodium salt of certain fatty acids. The importance of common salt to animal nutrition has been recognized since prehistoric times. Among the many compounds that are of the greatest industrial importance are common salt (NaCl), soda ash (Na2CO3), baking soda (NaHCO3), caustic soda (NaOH), Chile saltpeter (NaNO3), diand tri-sodium phosphates, sodium thiosulfate (hypo, Na2S2O3 · 5H2O), and borax (Na2B4O7 · 10H2O). Seventeen isotopes of sodium are recognized. Metallic sodium is priced at about $575/kg (99.95%). On a volume basis, it is the cheapest of all metals. Sodium metal should be handled with great care. It should be kept in an inert atmosphere and contact with water and other substances with which sodium reacts should be avoided.
Uses
Sodium is used in the manufacture ofmany highly reactive sodium compounds andtetraethyllead, as a reducing agent in organicsynthesis, and as a catalyst in the productionof synthetic rubber. It is also used in makingsodium lamp and photovoltaic cells.
Uses
This soft silvery metal occurring as chlorine in seawater was first isolated as an element by Humphry Davy in 1807. Sodium is one of the essential elements required by living organisms and it is highly reactive oxidizing in air and reacting with water. Sodium chloride was the first halide to be combined with silver for photographic purposes. Many of the sodium compounds were also used in gold toning baths. Some of them are included here.
Uses
manufacture of sodium Compounds, such as the cyanide, azide, peroxide, etc.; manufacture of tetraethyllead; manufacture of refractory metals; in org syntheses; for photoelectric cells; in sodium lamps; as catalyst for many polymerization reactions. Alloyed with potassium in heat transfer media.
Uses
Sodium is used in both low-pressure and high-pressure sodium vapor lamps. The low-pressure arc uses just a small amount of Na along with some neon for a starter. The lamp is economical and bright. The illumination with its single yellow color (electromagnetic frequency) makes it difficult for us to recognize other colors. In addition to sodium, the high-pressure lamp uses mercury, which provides a more natural color rendition of light. The very bright light of sodium-mercury lamps makes them ideal for use in sports stadiums and highways. Because the melting point of sodium metal is about 98 C (a bit lower than the boiling point of water), it is heated into a liquid phase and then transported in rail tank cars, where it cools and solidifies. Because sodium has a high specific heat rating, a major use is as a liquid coolant for nuclear reactors. Even though sodium (both solid and liquid) is extremely reactive with water, it has proven safe as a coolant for nuclear reactors in submarines. The natural deposits are located in northern Chile, which was the original source for many years. Of course, the most common use is everyday table salt, sodium chloride (NaCl).
Preparation
Sodium metal is produced commercially today by the electrolysis of fused sodium
chloride in a Downs cell. The Castner cell was the first commercially successful cell and
remained the principal source of sodium metal from about 1891 until being superseded by
the Downs cell6. This latter cell, introduced at duPont's Niagara Falls plant about 1921,
consists of a steel, refractory-brick-lined vessel with a graphite anode projecting upward from the bottom and a cast steel cathode surrounding the anode with an electrode spacing
of about 4 cm. The electrolyte is a eutectic mixture of roughly 40 % sodium chloride and
60 % calcium chloride, chosen so that the melting point of the mixed salt system is about
580°C, the operating temperature of the cell. The voltage drop across the cell is about
7 V and the current efficiency is roughly 85 %. As current flows through the molten salt
mixture, chlorine is liberated at the anode, and both sodium metal and calcium metal are
formed at the cathode; an iron gauze diaphragm between the electrodes prevents recombination.
The chlorine vapors from the anode flow overhead to a nickel collector dome
under slight vacuum, whence it is led to the chlorine purification and collection system.
The cathode product, a solution of calcium metal in liquid sodium, floats on the molten
salt bath and ascends through a vertical riser pipe into a collector vessel. As the solution
rises in the pipe, it is cooled to a temperature where calcium metal crystallizes, falls back into
the cell, and there reacts with the electrolyte. The sodium metal is filtered at 105-110°C to
remove calcium metal as well as small amounts of oxides and chlorides.
Many other methods for producing sodium metal have been devised, including electrolysis
of sodium carbonate, borate, nitrate, etc., but only the Castner-type cell based on
electrolysis of sodium hydroxide has had any appreciable commercial success. Today,
only the Downs-type cell is used commercially to produce sodium metal in most countries;
a few Castner-type cells are still operating, but total production is quite small.
Production Methods
Sodium is an essential element needed for all organic life. Sodium is produced commercially through the electrolysis of liquid sodium chloride mixed with calcium chloride in a Downs Cell. Very pure sodium can be isolated by the thermal decomposition of sodium azide (NaN3). Sodium, in its metallic form, can be used to refine some reactive metals, such as zirconium and potassium, from their compounds and is very important in making esters.
Definition
sodium: Symbol Na. A soft silveryreactive element belonging to group1 (formerly IA) of the periodic table(see alkali metals); a.n. 11; r.a.m.22.9898; r.d. 0.97; m.p. 97.8°C; b.p.882–889°C. Sodium occurs as thechloride in sea water and in the mineralhalite. It is extracted by electrolysisin a Downs cell. The metal isused as a reducing agent in certainreactions and liquid sodium is also acoolant in nuclear reactors. Chemically,it is highly reactive, oxidizingin air and reacting violently withwater (it is kept under oil). It dissolvesin liquid ammonia to formblue solutions containing solvatedelectrons. Sodium is a major essentialelement required by living organisms.The element was first isolatedby Humphry Davy in 1807.
General Description
Sodium,Na, melts at 97.8°C and boils at 892°C. It is silver-white in color, is soft and malleable, and oxidizes in air. When exposed to air, a silvery soft metal that becomes grayish white upon. It occurs naturally only in the forms of its salts. Shipped as a solid or molten liquid. Burns violently with explosions that may spatter the material. Sodium is used as a chemical intermediate. and in pharmaceuticals, petroleum refining and metallurgy, electric power cable, Sodium lamps, other chemicals.
Air & Water Reactions
May ignite spontaneously in air. Reacts violently with water to give Sodium hydroxide and hydrogen, which ignites spontaneously [Merck, 11th ed. 1989)]. The ignition temperature of Sodium in air depends on the area of surface exposed: vapor ignites at room temperature; droplets at about 250°F; an agitated pool at 400°F. In the absence of moisture and hydrogen, the reaction is insignificant [Mellor 2 Supp. 2:440 1961].
Reactivity Profile
Sodium is a powerful reducing agent. Reacts with incandescence with boron trifluoride [Merck 11th ed. 1989]. Reacts explosively with maleic anhydride [Chem Safety Data Sheet SD-88 1962; Chem. Haz. Info. Series C-71 1960]. Explodes on contact with bromoazide. Mixtures with any of the following produce a strong explosion on impact: aluminum bromide, aluminum chloride, aluminum fluoride, ammonium chloride, antimony(III) bromide, antimony(III) chloride, antimony(III) iodide, arsenic(III) chloride, arsenic(III) iodide, bismuth(III) bromide, bismuth(III) chloride, bismuth(III) iodide, boron tribromide, carbon tetrachloride, chromium(IV) chloride, cobalt(II) bromide, cobalt(II) chloride, copper(II) chloride, iron(II) chloride, iron(III) bromide, iron(II) iodide, iodine bromide, manganese(II) chloride, mercury(II) bromide, mercury(II) chloride, mercury(II) fluoride, mercury(II) iodide, mercury(I) chloride, silicon tetrachloride, silver fluoride, tin(IV) chloride, tin(IV) iodide (with sulfur), tin(II) chloride, sulfur dibromide, sulfur dichloride, thallium(I) bromide, vanadium pentachloride, phosphorus pentachloride, phosphorus tribromide, and zinc bromide [Mellor 2 Supp. 2:497 1961]. Reacts with ammonium nitrate to form a yellow explosive substance, thought to be diSodium nitrite [Mellor 8: Supp. 1 546 1964]. Reduces heated bismuth(III) oxide to the metal; the reaction is accompanied by incandescence [Mellor 9:649 1946-47]. Reacts, if finely divided, with bromine with luminescence. Burns spontaneously in moist chlorine. Reacts at room temperature with iodine [Mellor 2 Supp. 1:848 1956]. Reacts explosively with Dry Ice if the two are brought together by impact [Mellor 2 Supp. 2:468 1961]. Forms explosive mixtures with chlorinated hydrocarbons [Chem. Eng. News 26:2604 1948]. Explodes on contact with hydrochloric acid [Mellor 2:469 1946-47]. Explodes with aqueous hydrofluoric acid [Mellor 2:469 1946-47]. Ignites spontaneously in contact with dilute nitric acid [Mellor 2:470 1946-47]. Reacts with dilute sulfuric acid with explosive violence [Mellor 2:470 1946-47]. Sodium ignites on contact with hydroxylamine. (Mellor, 1940, Vol. 8, 292.)
Hazard
Sodium as the elemental metal is very dangerous because of its extreme electropositivenature, particularly when it comes in contact with moist air, water, snow, or ice or otheroxidizing agents. It readily gives up electrons to electronegative atoms (nonmetals). In thesereactions, it releases hydrogen gas with enough heat to explosively ignite the hydrogen.
Numerous sodium compounds are hazardous as carcinogens (cancer-causing) and astoxins (poisons) in plants and animals. On the other hand, we benefit greatly from the manycompounds containing the element sodium. We could not live without it.
Health Hazard
Sodium reacts with the moisture on skin and other tissues to form highly corrosive sodium hydroxide. Contact of metallic sodium with the skin, eyes, or mucous membranes causes severe burns; thermal burns may also occur due to ignition of the metal and liberated hydrogen.
Fire Hazard
Sodium spontaneously ignites when heated above 115 °C in air that has even modest moisture content, and any sodium vapor generated is even more flammable. Sodium reacts violently on contact with water and often ignites or explodes the hydrogen formed. Sodium fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or ''Met- L-X ? " type solids. Water or CO 2 extinguishers must never be used on sodium fires.
Flammability and Explosibility
Sodium spontaneously ignites when heated above 115 °C in air that has even modest moisture content, and any sodium vapor generated is even more flammable. Sodium reacts violently on contact with water and often ignites or explodes the hydrogen formed. Sodium fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or "Met- L-X ?" type solids. Water or CO2 extinguishers must never be used on sodium fires.
Agricultural Uses
Sodium (Na) is a silvery reactive element belonging to
Group 1 (formerly IA) of the Periodic Table.
It is not an essential element for any crop (including salt
marsh plants), but it is useful in certain biological
processes. Some crops grow better with sodium which is
absorbed as an ion.
Sodium influences water retention in sugar beet and
increases drought resistance. The supportive role of
sodium is not clear in some plants; however, in crops like
celery, marigold, sugar beet, turnip, etc., it increases the
amount of water held by a unit dry weight of the leaf
tissue and increases the succulence of the plant (which is
why these plants have a greater drought resistance and
increased leaf area.) Sugar beet and marigold in western
Europe, for instance, need a good supply of sodium for
satisfactory yields. In potassium deficient soils, sodium
helps the growth of crops like barley and prevents the
accumulation of other toxic cations, because deficiency
of one cation leads to accumulation of the other.
Sodium concentration varies widely from 0.01 to
10% in leaves. Sugar beet petioles frequently contain the
upper end of the range. In low-sodium soils, beet leaves
are dark green, thin and dull in hue and exhibit interveinal necrosis similar to that resulting from potassium
deficiency.
Sodium is essential for halophytic plants. Plants that
possess the C4 dicarboxylic acid photosynthetic pathway,
require sodium as an essential nutrient. Sodium has a role
in inducing crassulacean acid metabolism that is
responsible for water stress. The lack of sodium causes
certain plant species to shift their carbon dioxide fucation
pathway from C4 to C3. Water economy in plants seems to
be related to the C4 dicarboxylic photosynthetic pathway
of plants in fine textured soils. The benefits of sodium are
high when potassium is deficient.
The sodium demand of the crops is independent of,
and perhaps greater than, their potassium demand. The
important sodium-containing fertilizers are potassium
fertilizers with a wide ranging content of sodium
chloride, sodium nitrate, rhenania phosphate and
multiple nutrient fertilizers with sodium salts.
Sodium nitrate is available as a natural product, Chile
saltpeter, which contains trace amounts of
micronutrients, like boron. Synthetically, it is made from
nitric acid and sodium hydroxide.
The presence of sodium in soils is restricted to arid
and semi-arid regions. It is one of the most loosely held
metallic ions and is readily lost in leaching water. In fine
textured soils, sodium accumulation inhibits plant growth.
A high concentration of sodium is undesirable in
water as sodium is adsorbed on cation exchange sites,
causing soil aggregates to break down, sealing the soil
pores, and making it impermeable to water flow. Sodium
adsorption ratio (SAR) is used to estimate the
exchangeable percent sodium of soil; a low value
indicates low sodium content.
In sodic soils, exchangeable sodium is above 15 % and
its adsorption rate (SAR) is 13. The permeability is the
limiting factor in the reclamation of sodic soil. A high salt
content in water keeps sodic soils flocculated (joining of
colloidal particles to form clusters) and the floccules are
highly porous and allow penetration of the leaching
waters. Thus, the first water used for leaching may be
moderately salty. Sodic soil with low salt concentrations
readily loses its structure because it allows soil colloids
(clay and humus) to disperse into individual hydrated
particles.
Some of the effects ascribed to sodium may also be
due to the chloride ion in sodium chloride. In addition to
toxicity due to high concentrations of sodium and
chloride, sodium chloride affects plant growth because of
the osmotic effect which increases the potential forces
that hold water in the soil and makes it difficult for the
plant roots to extract moisture.
Fine textured clayey soils with low exchangeable
sodium (10%) and sandier soils with 20% exchangeable
sodium experience dispersion damage. Colloid dispersal
makes the soil impermeable to water and affects plant
growth. The impermeability to water causes soils to form
hard surface cmsts when dry. Uncorrected sodicity
makes most soils inhospitable to vegetation and agriculture.
Pharmaceutical Applications
Sodium has atomic number 11 and has the symbol Na, derived from the Latin name natrium. Sodium ions (Na+) are soluble in water and therefore present in large quantities in the oceans. Na+ is also part of minerals and an essential element for all animal life. The main biological roles of sodium ions are the maintenance of body fluids in humans and the functioning
of neurons and transmission of nerve impulses. Na+ is an important electrolyte and a vital component of the
extracellular fluid. Therefore, one of its roles is to maintain the fluid in the human body via osmoregulation,
a passive transport mechanism. Na+ ions also play a crucial role in the contraction of muscles
and in the mode of action of several enzymes. In the human body, Na+ is often used to actively build up an
electrostatic potential across membranes, with potassium ions (K+) being the counter-ion.
The build-up of an electrostatic potential across cell membranes is important to allow the transmission of
nerve impulses.
Sodium is an essential mineral for the human body and crucial for the regulation of the body fluid via its osmosis activity. Sodium ions account for over 90% of all ions in the plasma and in the interstitial fluid, which are involved in osmosis processes. Furthermore, it is the most abundant cation in the extracellular fluid, and therefore the Na+ content controls the extracellular volume. In particular, the kidneys play an important role in regulating the fluid level of the body as well as the filtration, secretion and re-absorption of Na+ in the nephrons, the functional unit of the kidney. Na+ ions are used in the human body to establish osmotic gradients, which in turn is crucial to control the water balance. Furthermore, decreases in blood pressure and in Na+ concentrations are sensed by the kidneys, and hormones (e.g. renin, antidiuretic hormones (ADHs), atrial natriuretic peptide) are released that control the blood pressure, osmotic balances and water-retaining mechanisms.
Safety Profile
Metahc sodtum reacts exothermally with the moisture of body or tissue surfaces, causing thermal and chemical burns. Sodium in elemental form is highly reactive. Sodium reacts violently with water to form sodium hydroxide. A very dangerous fire hazard when exposed to heat and moisture. Under the appropriate condttions, it can react violently with moisture, air, ALBr3, dcl3, AlF3, NH4 chlorocuprate, NH4NO3, SbBr3, SbCl3, SbI3, AsCl3, Ash, Bil3r3, BiCl3, BiI3, Biz03, BBr3, bromoazide, Con, CO + NH3, cCl4, Cl2, ClF3, CrCl4, Cr03, CoBr, CoCl, CuCl2, CuO,FeBrs, FeCL, FeBr2, FeCl2, FeI2, hydrazine hydrate, H202, H2S, HCl, HF, F2, 1,2dichloroethylene, dichloromethane, Brz, hydroxylamine, iodine, iodine monochloride, iodine pentafluoride, lead oxide, maleic anhydride, manganous chloride, mercuric bromide, mercuric chloride, mercuric fluoride, mercuric iodide, mercurous chloride, mercurous oxide, methyl chloride, molybdenum trioxide, monoammonium phosphate, nitric acid, nitrogen peroxide, nitrosyl fluoride, nitrous oxide, phosgene, phosphorus, phosphorus pentafluoride, phosphorus pentoxide, phosphorus tribromide, phosphorus trichloride, phosphoryl chloride, potassium oxides, potassium ozonide, potassium superoxide, selenium, silicon tetrachloride, silver bromide, silver chloride, silver fluoride, silver iodide, sodium peroxide, stannic chloride, stannic iodide with sulfur, stannic oxide, stannous chloride, sulfur, sulfur dibromide, sulfur dichloride, sulfur dioxide, sulfuric acid, tellurium, tetrachloroethane, thallous bromide, thiophosphoryl bromide, trichlorethylene, vanadium pentachloride, vanadyl chloride, zinc bromide, any oxidizing material. Decomposes moisture to evolve hydrogen and heat. Reacts exothermally with halogens, acids, and halogenated hydrocarbons. flammable in air. Can be safely stored under liquid hydrocarbons. Dangerous explosion hazard when exposed to moisture in any form!! Keep away from water at all times!! When heated in air it emits toxic fumes of sodium oxide. Reacts with water or steam to produce heat, hydrogen, and flammable vapors. Can react vigorously to explosively with oxidizing materials. To fight fEe, use soda ash, dry sodium chloride, or graphite, in order of preference. When heated to decomposition it emits toxic fumes of NazO. See also SODIUM HYDROXIDE and HYDROGEN. Heated sodium is spontaneously
Potential Exposure
A potential danger to those involved in tetra-alkyl lead manufacture using lead-sodium alloy as a reactant; those using sodium as a liquid metal coolant, as a catalyst, or in the manufacture of sodium hydride, borohydride, or peroxide.
Environmental Fate
Elemental sodium that is released into the environment reacts almost immediately with water to form sodium hydroxide and hydrogen gas. Even small quantities of metallic sodium can be explosive when brought into contact with sources of water; the formation sodium hydroxide raises the local pH and is extremely caustic. Sodium cations formed from this reaction are rapidly absorbed into the surrounding environment to form a large variety of salts.
storage
Safety glasses, impermeable gloves, and a fire-retardant laboratory coat should be worn at all times when working with sodium, and the metal should be handled under the surface of an inert liquid such as mineral oil, xylene, or toluene. Sodium should be used only in areas free of ignition sources and should be stored under mineral oil in tightly sealed metal containers under an inert gas such as argon.
Shipping
UN1428 Sodium, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. Note: Finely divided sodium is pyrophoric.
Purification Methods
The metal is placed on a coarse grade of sintered-glass filter, melted under vacuum and forced through the filter using argon. The Pyrex apparatus is then re-evacuated and sealed off below the filter, so that the sodium could be distilled at 460o through a side arm and condenser into a receiver bulb which is then sealed off [Gunn & Green J Am Chem Soc 80 4782 1958]. EXPLODES and IGNITES in water.
Toxicity evaluation
The formation of hydroxide ion following reaction of pure sodium with water in biological tissues is the primary mechanism of toxicity for sodium metal. The presence of the strong base raises the local pH and can lead to burns and/or irritation of the affected area.
Incompatibilities
A strong reducing agent. A dangerous fire hazard when exposed to heat and moisture. Violent reaction with water, forming NaOH. Violent reaction with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. halogenated hydrocarbons; phosphorus and phosphorus compounds; sulfur and sulfur compounds; and many other chemicals.
Waste Disposal
Incineration with absorption of oxide fumes.
Sodium Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
Related articles
- Is sodium a metal or nonmetal?
- Sodium is a metal. Firstly, from the periodic table we can see that sodium (Na) has an atomic number of 11 and is a member of ....
- Mar 8,2024
- How many neutrons, protons and electrons does a sodium atom have?
- A neutron is a neutral subatomic particle that, in conjunction with protons, makes up the nucleus of every atom except ordinar....
- Mar 6,2024
7440-23-5(Sodium)Related Search:
1of4