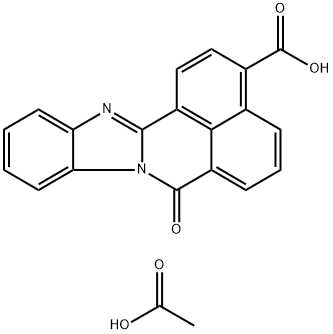
STO-609 (acetate)
- CAS No.
- 1173022-21-3
- Chemical Name:
- STO-609 (acetate)
- Synonyms
- CS-2405;PubChem ID: 16760660;STO-609;STO 609;STO609;7-oxo-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinoline-3-carboxylic acid;7H-Benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid, 7-oxo-, acetate (1:1);7-Oxo-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinoline-3-carboxylic Acid Acetate (1:1)
- CBNumber:
- CB53152750
- Molecular Formula:
- C21H14N2O5
- Molecular Weight:
- 374.35
- MDL Number:
- MOL File:
- 1173022-21-3.mol
- MSDS File:
- SDS
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
STO-609 (acetate) price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S1318 | STO-609-acetic acid ≥95%(HPLC),solid | 1173022-21-3 | 5mg | $204 | 2024-03-01 | Buy |
| Sigma-Aldrich | S1318 | STO-609-acetic acid ≥95% (HPLC), solid | 1173022-21-3 | 25MG | $643 | 2024-03-01 | Buy |
| Cayman Chemical | 15325 | STO-609 (acetate) ≥98% | 1173022-21-3 | 1mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 15325 | STO-609 (acetate) ≥98% | 1173022-21-3 | 5mg | $86 | 2024-03-01 | Buy |
| Cayman Chemical | 15325 | STO-609 (acetate) ≥98% | 1173022-21-3 | 10mg | $153 | 2024-03-01 | Buy |
STO-609 (acetate) Chemical Properties,Uses,Production
Description
STO-609 (1173022-21-3) is a selective inhibitor of Ca2+-calmodulin-dependent protein kinase kinase (Ki = 80 and 15 ng/ml for inhibition of CaM-KKα and CaM-KKβ respectively).1 Binds to the? ATP-binding site.2 Displays > 80-fold selectivity over CaMK1, CaMK2, CaMK4, MLCK, PKC, PKA and p42 MAPK. Important tool for probing distinct CaMK pathways in LTP.3 Reduces starvation-induced autophagosomal membrane formation.4 Reverses age-associated decline in bone mass.5 Stimulates osteoblast formation, inhibits osteoclast differentiation.6
Uses
STO-609 is a cell-permeable inhibitor of calcium/calmodulin-dependent kinase kinases (CaMKK) isoforms CaMKKα and CaMKKβ. It is used to evaluate the action of CaMKK isoforms both in vitro and in cells.
General Description
STO-609 reduces tumorigenicity of liver cancer cells in vivo. TO-609 reduces the activation of AMP (adenosine monophosphate)-activated protein kinase (AMPK) by ionomycin in a dose dependant manner.
Biochem/physiol Actions
Selective Ca2+/Calmodulin-dependent protein kinase kinase (CaM-KK) antagonist. Inhibits CamKKa and CaMKKb with Ki = 80 and 15 ng/mL, respectively. Does not inhibit downstream CaM kinases (CaMKI and CaMKIV).
storage
Room temperature (desiccate)
References
1) Tokumitsu?et al. (2002), STO-609, a Specific Inhibitor of the Ca2+/Calmodulin-dependent Protein Kinase Kinase;?J. Biol. Chem. 277 15813 2) Tokumitsu?et al. (2003) A single amino acid difference between alpha and beta Ca2+/calmodulin-dependent protein kinase kinase dictates sensitivity to the specific inhibitor, STO-609;?J. Biol. Chem. 278 10908 3) Redondo et al. (2010) Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation;?J. Neurosci. 30 4981 4) Pfisterer et al. (2011) Ca+2/calmodulin –dependent kinase (CaMK) signaling via CaMKI and AMP-activated protein kinase contributes to the regulation of WIPI-1 ar the onset of autophagy; Mol. Pharmacol. 80 1066 5) Pritchard et al. (2015) Inhibition of CaMKK2 reverses age-associated decline in bone mass; Bone, 75 120 6)Cary?et al. (2013) Inhibition of Ca+2/Calmodulin-dependent protein kinase kinase 2 stimulates osteoblast formation and inhibits osteoclast differentiation;?J. Bone Miner. Res. 28 1599 7) Matsukawa et al. (2017) Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance; Sci. Rep., 7 44799
STO-609 (acetate) Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32686 | 60 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5738 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| Shanghai Lollane Biological Technology Co.,Ltd. | 021-52996696,15000506266 15000506266 | China | 4153 | 55 |