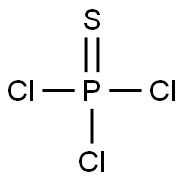THIOPHOSPHORYL CHLORIDE
- CAS No.
- 3982-91-0
- Chemical Name:
- THIOPHOSPHORYL CHLORIDE
- Synonyms
- Phosphorothioyl trichloride;thiophosphoryl;tl262;NSC 158334;AURORA KA-1089;fosforthiochlorid;Thiophosphoryl chlor;thiochloridfosforecny;Thiophosphorylchlorid;PHOSPHORSULFOCHLORIDE
- CBNumber:
- CB6115317
- Molecular Formula:
- Cl3PS
Lewis structure

- Molecular Weight:
- 169.4
- MDL Number:
- MFCD00011504
- MOL File:
- 3982-91-0.mol
| Melting point | -35 °C (lit.) |
|---|---|
| Boiling point | 125 °C (lit.) |
| Density | 1.668 g/mL at 25 °C (lit.) |
| vapor pressure | 21.9hPa at 25℃ |
| refractive index |
n |
| solubility | benzene: soluble(lit.) |
| form | liquid |
| color | colorless |
| Specific Gravity | 1.668 |
| Water Solubility | hydrolyzes in H2O, forming H3PO4, HCl, H2S; hydrolyzes rapidly in alkaline solutions; soluble benzene, CCl4, CS2, chloroform [MER06] |
| Sensitive | moisture sensitive |
| Merck | 14,7356 |
| Dielectric constant | 2.5(25℃) |
| Stability | Moisture Sensitive |
| CAS DataBase Reference | 3982-91-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | II99F8594N |
| EPA Substance Registry System | Phosphorothioic trichloride (3982-91-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H314-H330-H335 |
| Precautionary statements | P260-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T+ |
| Risk Statements | 22-26-34-52/53 |
| Safety Statements | 26-28-36/37/39-45-61 |
| RIDADR | UN 1837 8/PG 2 |
| WGK Germany | 2 |
| RTECS | XN2930000 |
| F | 21 |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28273985 |
THIOPHOSPHORYL CHLORIDE Chemical Properties,Uses,Production
Chemical Properties
clear colourless to slightly yellowish liquid
Uses
Thiophosphoryl chloride was used in the synthesis of O-ethyl dichlorothiophosphate.
General Description
A colorless fuming liquid. Boiling point 257°F (125 °C). Irritates the eyes and mucous membranes. Corrosive to metals and tissue.
Air & Water Reactions
Fumes in air. Decomposes in water to form phosphoric acid and hydrochloric acid (hydrogen chloride). Both substances are corrosive to metal or tissue. Can also form hydrogen sulfide (H2S), a toxic flammable gas, in reaction with water [AAR 1991].
Reactivity Profile
THIOPHOSPHORYL CHLORIDE is acidic. Incompatible with bases (including amines), with strong oxidizing agents, and with alcohols. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Strong irritant to skin and tissue.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by inhalation. Moderately toxic by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Explosive reaction with methylmagnesium iodlde. Explosive reaction with pentaerythritol + heat. Reacts with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits highly toxic fumes of POx, SOx, and Cl-.
Purification Methods
Possible impurities are PCl5, H3PO4, HCl and AlCl3. Gently mix it with H2O to avoid a heavy emulsion; the product decoulorises immediately and settles to the bottom layer. It is soluble in *C6H6 and CCl4. [Duval Inorg Synth IV 73 1953.] HARMFUL VAPOURS.
THIOPHOSPHORYL CHLORIDE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of7
3982-91-0(THIOPHOSPHORYL CHLORIDE)Related Search:
1of4