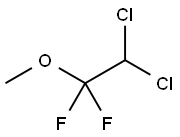
methoxyflurane
- CAS No.
- 76-38-0
- Chemical Name:
- methoxyflurane
- Synonyms
- MOZ;MOF;KAT8;hMOF;MYST1;KAT6A;MYST3;ZNF220;DA-759;RUNXBP2
- CBNumber:
- CB6314297
- Molecular Formula:
- C3H4Cl2F2O
- Molecular Weight:
- 164.97
- MDL Number:
- MFCD00040144
- MOL File:
- 76-38-0.mol
- MSDS File:
- SDS
| Melting point | -36°C |
|---|---|
| Boiling point | 103 °C |
| Density | 1.4262 |
| refractive index | 1.386 |
| Flash point | 37°C |
| storage temp. | -70°C |
| solubility | Chloroform (Sparingly) |
| form | liquid |
| color | Clear |
| Water Solubility | Miscible with alcohol, acetone, chloroform, ether, fixed oils and benzene. Immiscible with water. |
| Merck | 14,5994 |
| BRN | 1737766 |
| Exposure limits | No exposure limit is set. Based on comparison with related compounds, a TLV-TWA of 675 mg/m3 (100 ppm) is recommended. |
| Stability | Stability |
| CAS DataBase Reference | 76-38-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 30905R8O7B |
| NCI Drug Dictionary | methoxyflurane |
| ATC code | N02BG09 |
| EPA Substance Registry System | Methoxyflurane (76-38-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H319-H341 | |||||||||
| Precautionary statements | P210-P241-P308+P311 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10 | |||||||||
| Safety Statements | 23-24/25 | |||||||||
| RIDADR | 3271 | |||||||||
| RTECS | KN7820000 | |||||||||
| Hazard Note | Irritant | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2909191800 | |||||||||
| Toxicity | One of the most potent of the inhalational anesthetics, having a very high blood-gas partition coefficient and low vapor pressure at room temperature. Methoxyflurane is metabolized to a great extent (about 50-70%) in the liver and, as a consequence, there may be release of high concentrations of fluoride, sufficient to exceed the threshold for renal damage. Its use for sustained anesthesia is limited because of this renal toxicity and was discontinued around 1980. | |||||||||
| NFPA 704 |
|
methoxyflurane price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1418004 | Methoxyflurane United States Pharmacopeia (USP) Reference Standard | 76-38-0 | 0.5ml | $453 | 2024-03-01 | Buy |
| Alfa Aesar | L17285 | 2,2-Dichloro-1,1-difluoroethyl methyl ether, 97% | 76-38-0 | 5g | $91.4 | 2023-06-20 | Buy |
| Alfa Aesar | L17285 | 2,2-Dichloro-1,1-difluoroethyl methyl ether, 97% | 76-38-0 | 25g | $347 | 2023-06-20 | Buy |
| Usbiological | 486453 | MYST3 | 76-38-0 | 100ul | $499 | 2021-12-16 | Buy |
| TRC | M262675 | Methoxyflurane | 76-38-0 | 10g | $1320 | 2021-12-16 | Buy |
methoxyflurane Chemical Properties,Uses,Production
Description
Methoxyflurane, 2,2-dichloro- 1,1- difluoro-1-methoxyethane,is a colorless liquid with a fruity odor. It is produced industrially by the addition of methanol to 1,1-dichloro- 2,2-difluoroethylene in the presence of sodium methoxide .
Chemical Properties
colourless liquid
Originator
Penthrane,Abbott,US,1962
Uses
Methoxyflurane is used as a clinical anesthesia (inhalation).
Uses
2,2-Dichloro-1,1-difluoroethyl methyl ether is used as a lipid soluble anesthetic agent. It is a neuromuscular blocking agent given concurrently to get the desired degree of muscular relaxation. Further, it is a powerful analgesic agent.
Uses
Methoxyflurane is a very potent and highly lipid soluble anesthetic agent. Methoxyflurane causes deep sedation and it has been used as a patient controlled analgesic for painful procedures in children. Methoxyflurane is a significant respiratory depressant.
Definition
ChEBI: An ether in which the two groups attached to the central oxygen atom are methyl and 2,2-dichloro-1,1-difluoroethyl.
Manufacturing Process
Into a reactor equipped with agitator and temperature control jacket is charged approximately 100 lb (about 3 lb mols) of methanol, technical. This methanol is used in excess, and so it is both a reactant and a solvent in the synthesis.
Approximately 1 US gallon of ion exchange resin beads wet with methanol is then added to the methanol. This is in the hydroxide form with at least 0.7 milliequivalent OH- per milliliter of wet beads. Approximately 190 lb of 1,1- dichloro-2,2-difluoroethylene (about 1.44 lb mols) is then added to the reactor and, within it, to the 100 lb of methanol through a sparge pipe while the beads are kept in suspension by agitation. Coolant is run through the jacket of the reactor during this addition because the reaction is exothermic. The temperature in the reaction medium is kept at 10° to 20°C, to prevent side reactions and to minimize losses of the dichlorodifluoroethylene, which boils at 17°C. Reaction time is affected by the rate of heat removal and the reaction normally takes from 4 to 8 hours, using the stated quantities and conditions. After the dichlorodifluoroethylene is added, the resin is checked for residual alkalinity. If the resin is alkaline to phenolphthalein, it is assumed to have been of sufficient capacity and is removed from the CH3OCF2CHCI2-methanol mixture. If it is not alkaline to phenolphthalein, additional resin is added to insure complete reaction.
Essentially the same procedure can be carried out, employing as alkali any strongly alkaline substance, such as caustic soda in methanol solution. Control of the reaction rate may be accomplished by the rate of the addition of reactants and the amount of cooling applied to the reaction mixture. Agitation is employed to insure efficient contact of the reactants.
After removal of the resin catalyst, the excess methanol is extracted out of the mixture using three separate water washes, suitably of 25 gallons each. The water layer is decanted off, leaving product as an immiscible organic layer, after each wash. The 2,2-dichloro-1,1-difluoroethyl methyl ether containing intolerable unsaturated impurities may be purified and stabilized by a treatment with oxidizing agents such as air, oxygen, ozone, peroxy compounds, or other similar oxidizing agents, with subsequent removal of the decomposition or oxidation products and distilling if desired.
brand name
Penthrane (Abbott).
Therapeutic Function
Inhalation anesthetic
Biological Functions
Methoxyflurane (Penthrane) is the most potent inhalational agent available, but its high solubility in tissues limits its use as an induction anesthetic. Its pharmacological properties are similar to those of halothane with some notable exceptions. For example, since methoxyflurane does not depress cardiovascular reflexes, its direct myocardial depressant effect is partially offset by reflex tachycardia, so arterial blood pressure is better maintained. Also, the oxidative metabolism of methoxyflurane results in the production of oxalic acid and fluoride concentrations that approach the threshold of causing renal tubular dysfunction. Concern for nephrotoxicity has greatly restricted the use of methoxyflurane.
General Description
Methoxyflurane is a volatile liquid (bp=105°C) with a highblood:gas partition coefficient and thus a slow induction andprolonged recovery. Approximately 75% of the drug undergoesmetabolism yielding dichloroacetate, difluoromethoxyacetate,oxalate, and fluoride ions. The intrarenal inorganicfluoride concentration, as a result of renal defluorination, maybe responsible for the nephrotoxicity seen with methoxyflurane.Both the concentration of F- generated and the durationfor which it remained elevated were factors in the developmentof methoxyflurane nephrotoxicity. Methoxyfluranewas removed from the U.S. market in 2000 because of saferalternatives. Both isoflurane and enflurane produce less fluorideion upon metabolism than methoxyflurane.
General Description
Clear colorless liquid with a sweet fruity odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,2-DICHLORO-1,1-DIFLUOROETHYL METHYL ETHER may be sensitive to prolonged exposure to light.
Health Hazard
Methoxyflurane exhibited low to very lowacute toxicity via inhalation, slightly lowerthan that of ethrane. Oral toxicity was low tomoderate depending on the species. Inhala tion of its vapors at 1.5–2% by volumeconcentrations in air can cause anesthesia inhumans. The toxic symptoms are similar tothose of ethrane, and the target organs areprimarily the central nervous system, kidney,and liver. At subanesthetic concentrations of0.3–0.5% by volume in air, its exposure tohumans for 1 hour resulted in the onset oflow toxicity. The sites of biological effectswere in the kidney.
LC50 value, inhalation (mice): 17,500 ppm/2 hr
LD50 value, oral (mammals): 3600 mg/kg
The liquid may be an irritant to theeyes. The teratomeric properties of this com pound were observed in rats and mice. Thesymptoms were embryo deaths and develop mental abnormalities in the urogenital andmusculoskeletal systems.
No carcinogenic actions in animals orhumans have been reported. The histidinereversion–Ames test for mutagenicity wasinconclusive.
Fire Hazard
2,2-DICHLORO-1,1-DIFLUOROETHYL METHYL ETHER is combustible.
Clinical Use
Methoxyflurane is seldom used because of its propensity to cause renal toxicity. It is the most potent agent, and it has the highest solubility in blood. Induction and recovery would be expected to be slow. Chemically, it is rather unstable, and as much as 50% of an administered dose can be metabolized. Toxic metabolites significantly limit its utility as a general anesthetic.
Veterinary Drugs and Treatments
Methoxyflurane is an inhalant anesthetic, but it is rarely used today primarily due to its potential for causing nephrotoxicity, slow onset of action (a short-acting barbiturate is often used as an induction agent), and prolonged recovery time. However, it does produce some muscle relaxation and analgesia, even at relatively low concentrations and can be administered without a precision vaporizer as it will vaporize to a maximum of about 3%.
methoxyflurane Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
| CD Chemical Group Limited | +8615986615575 | info@codchem.com | China | 20356 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Shanghai Sinch Parmaceuticals Tech. Co. Ltd. | +86-21-54098501 | sales@sinch.com.cn | China | 11605 | 64 |
View Lastest Price from methoxyflurane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-09 | methoxyflurane USP/EP/BP
76-38-0
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- methoxyflurane USP/EP/BP
76-38-0
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited