ChemicalBook >> CAS DataBase List >>(1S,2R)-(-)-cis-1-Amino-2-indanol
(1S,2R)-(-)-cis-1-Amino-2-indanol
- CAS No.
- 126456-43-7
- Chemical Name:
- (1S,2R)-(-)-cis-1-Amino-2-indanol
- Synonyms
- (1S,2R)-1-AMINO-2,3-DIHYDRO-1H-INDEN-2-OL;(1S,2R)-1-AMINO-2-INDANOL;(1S,2R)-CIS-1-AMINO-2-INDANOL;(1S,2R)-(-)-1-AMINO-2-INDANOL;CIS-(1S,2R)-1-AMINO-2-INDANOL;(1S;3aR;(1S,2R) -(−KT-1-Amino-2-indanol-0001;(1S,2R)-1-AMINO-INDAN-2-OL
- CBNumber:
- CB6417161
- Molecular Formula:
- C9H11NO
- Molecular Weight:
- 149.19
- MDL Number:
- MFCD00216655
- MOL File:
- 126456-43-7.mol
- MSDS File:
- SDS
Last updated:2024-04-23 16:18:34
| Melting point | 118-121 °C(lit.) |
|---|---|
| alpha | -62 º (c=0.5, CHCl3) |
| Boiling point | 270.27°C (rough estimate) |
| Density | 1.0753 (rough estimate) |
| refractive index | 1.5760 (estimate) |
| RTECS | NK7525500 |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | soluble in Methanol |
| pka | 14.79±0.40(Predicted) |
| form | Powder |
| color | White to light beige |
| optical activity | [α]20/D 61°, c = 0.5 in chloroform |
| Water Solubility | slightly soluble |
| Sensitive | Air Sensitive |
| BRN | 4292559 |
| InChIKey | LOPKSXMQWBYUOI-BDAKNGLRSA-N |
| CAS DataBase Reference | 126456-43-7(CAS DataBase Reference) |
| FDA UNII | LU3GK925A8 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36-36/37/39 | |||||||||
| RIDADR | 3259 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10-23 | |||||||||
| TSCA | No | |||||||||
| HazardClass | 8 | |||||||||
| HS Code | 29052900 | |||||||||
| NFPA 704 |
|
(1S,2R)-(-)-cis-1-Amino-2-indanol price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 440833 | (1S,2R)-(?)-cis-1-Amino-2-indanol 99% | 126456-43-7 | 1g | $26 | 2024-03-01 | Buy |
| Sigma-Aldrich | 440833 | (1S,2R)-(?)-cis-1-Amino-2-indanol 99% | 126456-43-7 | 5g | $27.1 | 2024-03-01 | Buy |
| TCI Chemical | A1624 | (1S,2R)-(-)-1-Amino-2-indanol >98.0%(GC)(T) | 126456-43-7 | 1g | $30 | 2024-03-01 | Buy |
| TCI Chemical | A1624 | (1S,2R)-(-)-1-Amino-2-indanol >98.0%(GC)(T) | 126456-43-7 | 5g | $131 | 2024-03-01 | Buy |
| Alfa Aesar | H32066 | (1S,2R)-(-)-cis-1-Amino-2-indanol, 97% | 126456-43-7 | 5g | $146.65 | 2024-03-01 | Buy |
(1S,2R)-(-)-cis-1-Amino-2-indanol Chemical Properties,Uses,Production
Reaction
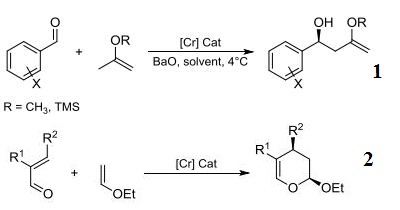
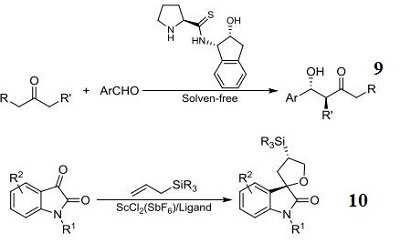
- Ligand component used in the chromium-catalyzed highly selective asymmetric ene reactions between aryl aldehydes and alkoxy- and silyloxyalkenes.
- Ligand component for the chromium-catalyzed highly enantioselective o inverse-demand hetero-Diels-Alder reactions of α,β-unsaturated aldehydes.
- Ligand component for the magnesium-catalyzed conjugate addition reaction of 1,3-dicarbonyl compounds to nitroalkenes.
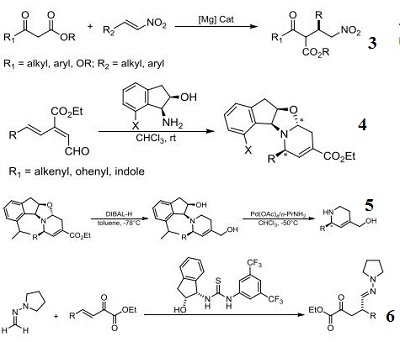
- Component for stereoselective asymmetric 6π-azaelectrocyclization through the reaction between the (E)-3-
- carbonyl-2,4,6-trienal compounds and the (-)-7-alkyl-cis-1-amino-2-indanol derivatives.
- Ligand component for palladium-catalzyed asymmetric azaelectrocyclization for the preparation of 2,4-
- disubstituted chiral 1,2,5,6-tetrahydropyridines.
- Component for organocatalytic conjugate addition of formaldehyde N,N-dialkylhydrazones to β,γ -Unsaturated α-keto esters.
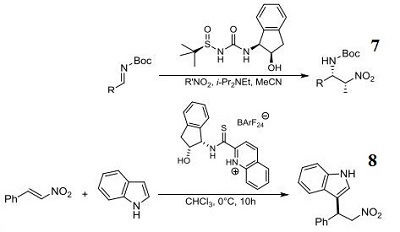
- N-Sulfinyl urea organocatalyst component for enantioselective aza-henry reaction.
- Component for organocatalytic enantioselective additions of indoles to nitroalkenes.
Chemical Properties
white to light yellow crystal powder
Uses
(1S,2R)-(-)-cis-1-Amino-2-indanol is used as a reagent in the synthesis of heterocyclic compounds as integrase inhibiting antiviral agents. It is also a key intermediate of the HIV protease inhibitor, Indinavir (I525000).
Uses
1S,2R)-(-)-cis-1-Amino-2-indanol may be used to prepare:
- (-)-1,2,5,6-Tetrahydropyridine by reacting with methyl (E,E)-4-oxo-2-[(2,6,6-trimethylcyclohex-1-enyl)vinyl}but-2-enoate.
- Oxazaborolidine catalysts, which can catalyze the asymmetric reduction of aromatic ketones with high enantioselectivity.
- (RS,1S,2R)-(-)-2,4,6-Trimethylbenzenesulfinic acid 1-(2,4,6-trimethylbenzenesulfonylamino)indan-2-yl ester.
General Description
(1S,2R)-(-)-cis-1-Amino-2-indanol is a main constituent of indinavir, a potent HIV (human immunodeficiency virus) protease inhibitor.
(1S,2R)-(-)-cis-1-Amino-2-indanol Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 391)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 2259 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shanghai Arbor Chemical Co., Ltd. | 021-60451682 | act@arborchemical.com | CHINA | 906 | 58 |
| Changzhou Ansciep Chemical Co., Ltd. | +86 519 86305871 | sales@ansciepchem.com | CHINA | 4242 | 58 |
View Lastest Price from (1S,2R)-(-)-cis-1-Amino-2-indanol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | (1S,2R)-(-)-cis-1-Amino-2-indanol
126456-43-7
|
US $5.00 / kg | 1kg | ≥99% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-23 | (1S,2R)-(-)-cis-1-Amino-2-indanol
126456-43-7
|
US $50.00 / kg | 1kg | 99.10% | 50000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2023-07-29 | (1S,2R)-(-)-cis-1-Amino-2-indanol
126456-43-7
|
US $100.00 / kg | 25kg | 99.99% | 200ton | Hebei Mojin Biotechnology Co., Ltd |
-

- (1S,2R)-(-)-cis-1-Amino-2-indanol
126456-43-7
- US $5.00 / kg
- ≥99%
- Jinan Finer Chemical Co., Ltd
-

- (1S,2R)-(-)-cis-1-Amino-2-indanol
126456-43-7
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- (1S,2R)-(-)-cis-1-Amino-2-indanol
126456-43-7
- US $100.00 / kg
- 99.99%
- Hebei Mojin Biotechnology Co., Ltd
126456-43-7((1S,2R)-(-)-cis-1-Amino-2-indanol)Related Search:
1of4
(1S,2R)-(-)-cis-1-Amino-2-indanol,99%
(1S, 2R)-Trans-1-AMino-2-indanol
(1S,2R)-(-)-1-Amino-2,3-dihydro-1H-inden-2-ol, (1S,2R)-(-)-1-Aminoindan-2-ol
(1S,2R)-1-Amino-2,3-dihydro-inden-2-ol
(1S,2R)-(-)-cis-1-Amino-2-indanol ,98%
(1S
2R)-(-)-cis-1-AMino-2-indanol
3aR
7aR)-1-((S)-1-(3-(tert-butyldiMethylsilyloxy)-3-Methylbutoxy)ethyl) -7a-Methylhexahydro-1H-inden-4(2H)-one
KT-1-Amino-2-indanol-0001
(1S,2R)-(-)-cis-1-Amino-2-indanol
(1S,2R)-(-)-cis-1-Amino-2-hydroxyindane
(1S,2R)-(-)-1-Amino-2-indanol, >=98%
(1S,2R)-1-Amino-2-indanol,99%e.e.
(1S,2R)-(-)-CIS-1-AMINO-2-HYDROXYINDANE
(1S,2R)-(-)-CIS-1-AMINO-2-INDANOL
(1S,2R)-(-)-CIS-1-AMINOINDAN-2-OL
(1S,2R)-(+)-CIS-1-AMINOINDAN-2-OL
(1S,2R)-(-)-1-AMINO-2-HYDROXYINDAN
(1S,2R)-1-AMINO-2-HYDROXYINDANE
(1S,2R)-1-AMINO-INDAN-2-OL
cis-(1S)-Amino-(2R)-indanol
(Is-Cis)L-Amino-2,3-Dihydro-Lh-Inden-2-Ol,IndinavirSulphate,
(IS-cis) l-Amino-2,3-Dihydro-lH-Inden-2-ol
CIS-(1S,2R)-1-AMINO-INDANE-2-OL
(1S,2R)-(1)-cis-1-Amino-2-indanol
(1S,2R)-(-)-cis-1-Aminoindan-2-ol,98%
(1S,2R)-(-)-CIS-1-AMINO-2-INDANOL 99%
(1R,2S)-(-)-cis-a-Amino-2-indanol
5R)-LGH447 dihydrochloride
(1S,2R)-(-)-cis-1-Amino-2-hydroxyindane 126456-43-7 (1S,2R)-(-)-cis-1-Amino-2-indanol
(1S,2R)-(-)-cis-1-Amino-2-indanol 126456-43-7
(1S,2R)-(-)-1-Amino-2-indanol >
1H-Inden-2-ol, 1-amino-2,3-dihydro-, (1S,2R)-
Indinavir Impurity 1 (Indinavir EP Impurity A)
(1S,2R)-(-)-cis-1-Amino-2-indanol USP/EP/BP
(1S,2R)-(-)-cis-1-Amino-2-indanol, 97%,
(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-aminium
(1S,2R)-CIS-1-AMINO-2-INDANOL
(1S,2R)-1-AMINO-2,3-DIHYDRO-1H-INDEN-2-OL
(1S,2R)-(-)-1-AMINO-2-INDANOL
(1S,2R)-1-AMINO-2-INDANOL
CIS-(1S,2R)-1-AMINO-2-INDANOL
(1S,2R) -(&minus
(1S,2R)-(-) -1-amino-2-indenol
126456-43-7
126456-43-9
C9H11NO
Amino Alcohols (Chiral)
Chiral Building Blocks
Hydrogenation
Organic Building Blocks
Chiral Catalysts, Ligands, and Reagents
Asymmetric Synthesis
Amino Alcohols
Chiral Nitrogen
organic alcohol
Amino Alcohols (Chiral)
Chiral Building Blocks