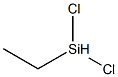
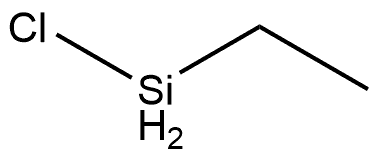
Ethyldichlorosilane
- CAS No.
- 1789-58-8
- Chemical Name:
- Ethyldichlorosilane
- Synonyms
- DICHLOROETHYLSILANE;ETHYLDICHLOROSILANE;dichloroethyl-silan;dichloro(ethyl)silicon;Dichloroethylsilane>Silane, dichloroethyl-;Monoethyldichlorosilane;Dichloroethylsilane,Ethyldichlorosilane
- CBNumber:
- CB6715098
- Molecular Formula:
- C2H6Cl2Si
- Molecular Weight:
- 129.06
- MDL Number:
- MFCD00053205
- MOL File:
- 1789-58-8.mol
| Melting point | -107°C |
|---|---|
| Boiling point | 74-76°C |
| Density | 1.089 g/mL at 20 °C(lit.) |
| refractive index |
n |
| Flash point | -9°C |
| storage temp. | Refrigerator |
| Specific Gravity | 1.093 |
| Odor | Sharp, hydrochloric acid-like; acrid. |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| CAS DataBase Reference | 1789-58-8(CAS DataBase Reference) |
| FDA UNII | I2TV9F1B6E |
| NIST Chemistry Reference | Ethyldichlorosilane(1789-58-8) |
| EPA Substance Registry System | Silane, dichloroethyl- (1789-58-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H260-H314-H334-H335 | |||||||||
| Precautionary statements | P210-P223-P231+P232-P261-P370+P378-P422 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 11-14/15-34-37 | |||||||||
| Safety Statements | 16-26-36/37/39-43-45 | |||||||||
| RIDADR | UN 1183 4.3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | VV3230000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| NFPA 704 |
|
Ethyldichlorosilane price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | D1846 | Dichloroethylsilane >97.0%(GC) | 1789-58-8 | 25g | $167 | 2022-04-27 | Buy |
| Chem-Impex | 40432 | Dichloroethylsilane,97%(GC) 97%(GC) | 1789-58-8 | 25G | $181.44 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD75596 | Dichloroethylsilane | 1789-58-8 | 2g | $196.8 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD75596 | Dichloroethylsilane | 1789-58-8 | 5G | $393.6 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FD75596 | Dichloroethylsilane | 1789-58-8 | 10g | $669.6 | 2021-12-16 | Buy |
Ethyldichlorosilane Chemical Properties,Uses,Production
Description
Ethyl dichlorosilane is a colorless liquid witha sharp, irritating odor. Molecular weight = 129.07;Boiling point = 75.5℃; Flash point =-1℃. Explosivelimits: LEL: 2.9%; UEL: unknown. Hazard Identification (based on NFPA-704 M Rating System): Health 3,Flammability 4, Reactivity 2 . Dangerously reactive withwater.
Chemical Properties
Ethyl dichlorosilane is a colorless liquid. Sharp, irritating odor
Chemical Properties
Colorless liquid.Readily hydrolyzed by moisture, with the liberation of hydrogen and hydrogen chloride.
Uses
Intermediate for silicones.
Application
Will form high-boiling polymeric by-products with aqueous work-up.
General Description
A colorless fuming liquid with a pungent odor. Flash point 30°F. Vapor and liquid may cause burns. Denser than water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Based on the properties of similar materials, there is the possibility that the reaction of Ethyldichlorosilane with water may be vigorous or violent. Products of the reaction include hydrogen chloride. The reaction generates heat and this heat may be sufficient to ignite the product. The chlorosilicon hydrides(ClxSiHy) are spontaneously flammable in air [NFPA 1991].
Reactivity Profile
Chlorosilanes, such as Ethyldichlorosilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Hazard
Flammable, dangerous fire risk. Strong irritant to eyes and skin.
Health Hazard
Inhalation irritates mucous membranes. Contact with liquid causes severe burns of eyes and skin. Ingestion causes severe burns of mouth and stomach.
Chemical Reactivity
Reactivity with Water Reacts vigorously, evolving hydrogen chloride (hydrochloric acid); Reactivity with Common Materials: Reaction with surface moisture will generate hydrogen chloride, which corrodes common metals; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flood with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by ingestion and inhalation. A severe irritant to skin, eyes, and mucous membranes. Corrosive. Dangerous fire hazard if exposed to heat, open flames, or powerful oxidizers. Will react with water or steam to produce heat and toxic and corrosive fumes. To fight fire, use foam, dry chemical, mist, spray. When heated to decomposition it emits toxic fumes of Cland phosgene. See also CHLOROSILANES.
Potential Exposure
This material is used in silicone polymer manufacture.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24- 48 h after breathing overexposure, aspulmonary edema may be delayed.
storage
(1) Color Code—Red: Flammability Hazard: Storein a flammable liquid storage area or approved cabinetaway from ignition sources and corrosive and reactivematerials. (2) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Ethyldichlorosilane must be stored to avoid contact with moistureand oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates) since violent reactions occur.Store in tightly closed containers in a cool, well-ventilatedarea away from water, steam, and moisture because toxic andcorrosive chloride gases, including hydrogen chloride can beproduced. Sources of ignition, such as smoking and openflames, are prohibited where ethyl dichlorosilane is handled,used, or stored. Metal containers involving the transfer of 5gallons or more of ethyl dichlorosilane should be groundedand bonded. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters. Useonly nonsparking tools and equipment, especially whenopening and closing containers of ethyl dichlorosilane.
Shipping
UN1183 Ethyldichlorosilane, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material; 8-Corrosive material
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Chlorosilanes react vigorously with bases and both organic and inorganic acids generating toxic and/or flammable gases. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous hydrogen. Attacks metals in the presence of moisture
Ethyldichlorosilane Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Win-Win Chemical CO., Limited | 0086-577-64498589 | sales@win-winchemical.com | CHINA | 998 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| WinWin Chemical CO., Limited | +86-0086-577-64498589 +86-15355981851 | sales@win-winchemical.com | China | 13854 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | cherry@crovellbio.com | China | 18455 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23931 | 58 |