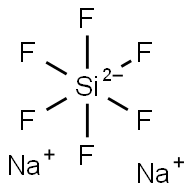
Sodium fluorosilicate
- CAS No.
- 16893-85-9
- Chemical Name:
- Sodium fluorosilicate
- Synonyms
- SODIUM SILICOFLUORIDE;SSF;SODIUM HEXAFLUOROSILICATE;ens-zemweevilbait;SODIUM FLUOSILICATE;Sodium flurosilicate;safsan;salufer;ent1,501;氟硅酸钠生产厂家价格
- CBNumber:
- CB7423572
- Molecular Formula:
- F6Na2Si
- Molecular Weight:
- 188.06
- MDL Number:
- MFCD00003491
- MOL File:
- 16893-85-9.mol
| Melting point | melts at red heat, decomposing [MER06] |
|---|---|
| Boiling point | 620℃[at 101 325 Pa] |
| Density | 2.68 g/mL at 25 °C(lit.) |
| vapor pressure | 0.01Pa |
| refractive index | 1.310 |
| solubility | insoluble in ethanol |
| form | Crystals |
| Specific Gravity | 2.68 |
| color | White |
| Odor | odorless |
| Water Solubility | Slightly soluble in water. Solubility increases with temperature. Insoluble in alcoholSlightly soluble in water. Insoluble in alcohol. |
| Merck | 14,8624 |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability | Stable. Incompatible with oxidizing agents, water. Moisture sensitive. |
| LogP | 0.001 |
| CAS DataBase Reference | 16893-85-9(CAS DataBase Reference) |
| EWG's Food Scores | 1-3 |
| FDA UNII | 806AV2E065 |
| EPA Substance Registry System | Sodium fluosilicate (16893-85-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331 | |||||||||
| Precautionary statements | P280-P301+P310+P330-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 23/24/25 | |||||||||
| Safety Statements | 26-45 | |||||||||
| RIDADR | UN 2674 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VV8410000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2826908090 | |||||||||
| Toxicity | LD in rabbits (mg F/kg): 76 intragastric; in rats (mg F/kg): 42 s.c. (Muehlberger); LD in guinea pigs (mg/kg): 250 orally, 500 s.c. (Waldbott) | |||||||||
| NFPA 704 |
|
Sodium fluorosilicate price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 250171 | Sodium hexafluorosilicate | 16893-85-9 | 500g | $58 | 2023-01-07 | Buy |
| Sigma-Aldrich | 250171 | Sodium hexafluorosilicate | 16893-85-9 | 2kg | $122 | 2023-01-07 | Buy |
| Alfa Aesar | 069106 | Sodium hexafluorosilicate, 99+% | 16893-85-9 | 500g | $50.3 | 2023-06-20 | Buy |
| Alfa Aesar | 069106 | Sodium hexafluorosilicate, 99+% | 16893-85-9 | 2kg | $116 | 2023-06-20 | Buy |
| Strem Chemicals | 93-1147 | Sodium hexafluorosilicate, 99% | 16893-85-9 | 100g | $29 | 2024-03-01 | Buy |
Sodium fluorosilicate Chemical Properties,Uses,Production
Outline
Sodium fluorosilicate appears as white crystal, crystalline powder or colorless hexagonal crystals. It is odorless and tasteless. Its relative density is 2.68; it has moisture absorption capability. It can be dissolved in a solvent such as ethyl ether but insoluble in alcohol. The solubility in acid is greater than that in water. It can be decomposed in alkaline solution, generating sodium fluoride and silica. After searing (300 ℃), it is decomposed into sodium fluoride and silicon tetrafluoride. It is poisonous!
In 25 degrees, the sodium fluorosilicate has its water solubility being 0.78%. Upon production, addition of an excess amount of sodium sulfate or sodium chloride can increase the concentration of sodium ions in the system, thereby increasing the solubility product of fluorine silicate and sodium ions, so that sodium fluoride can be subject to more complete precipitation.
Sodium fluorosilicate is the byproduct in the production of calcium phosphate or the production of fluoride salt in aluminum factory. It generally appears as a white crystalline powder with low solubility in water (being 1% or less at room temperature), and the water solubility increases slightly with increasing temperature. The aqueous solution of sodium fluorosilicate is acidic (pH3). This is due to that its hydrolysis product contains hydrofluoric acid. Reduction of the acidicity of the solution facilitates the hydrolysis of sodium fluorosilicate. When pH is lower than 3.5-3.55, the hydrolysis reaction is stabilized. When the pH is equal to 4, the hydrolysis reaction rate is significant. When the pH value is equal to 8-8.5, the sodium fluorosilicate can be completely hydrolyzed to exhibit as silica gel and precipitated out. Therefore, when sodium fluorosilicate is added to the water glass, in addition to hardening effect of precipitation of silicon dioxide gel caused by the neutralization of the sodium hydroxide in the solution of water glass, the sodium fluorosilicate itself is also a source of silica gel.
Reference quality standards
105 ℃ Loss of weight upon drying% 0.17%
Sodium fluorosilicate (based on dry mass) 99.50
Free acid (calculated based on HCL) 0.0075
Water insoluble matter 0.12%
Heavy metals (Pb) content% <0.02
% Fineness (through 250um sieve) 98.00
Appearance white powder
The role and purpose
Sodium fluorosilicate can be used as the insecticide in agriculture, the milky agent of enamel, the corrosion production agent of opal glass, wood preservatives and the raw material for the production of other kinds of fluoride as well as being applied to the smelting of beryllium and aluminum and pharmaceuticals, leather and rubber industry. In the refractory material, it can be used as the coagulant as the water glass binding agent. Since sodium fluorosilicate has a low water solubility, it reaction with the water glass is slow and gradual. This can not only boost the construction but also has a high density and strength of the cured products. It is most commonly used in the inorganic material binding with the water glass.
This information is edited by Xiongfeng Dai from Chemicalbook.
Toxicity
This product is toxic with stimulation effect on the respiratory organ. People of mistakenly oral poisoning will get severe symptoms of damage to the gastrointestinal tract with the lethal dose being 0.4~4g. During the working of the operator, they should wear the necessary protective equipment to prevent poisoning. Production equipment should be sealed and the workshop should be well ventilated.
Chemical Properties
It appears as colorless hexagonal crystals. It is odorless and tasteless. It has hygroscopicity. It can be dissolved in a solvent such as ethyl ether but insoluble in alcohol with its solubility in acid is being greater than in the water.
Production method
Calcium superphosphate byproduct method: use sulfuric acid and ground phosphate rock for production of superphosphate or fluorine-containing waste gas during extraction of phosphate with water absorbing silicon tetrafluoride to make it into fluorosilicate. When the concentration of fluorine acid solution reaches 8% to 10%, stand static for clarification and remove the impurities with the clarified fluorine silicate solution being added of sodium chloride (being excess of about 25%) for reaction of sodium fluorosilicate. Further go through centrifugation isolation, washing, air drying at a temperature below 300 ℃ and then pulverize to obtain the finished product of sodium fluorosilicate.
H2SiF6 + 2NaCl → Na2SiF6 + 2HCl
Category
Toxic substances.
Toxicity grading
Highly toxic.
Acute toxicity
Oral-mouse LD50: 70 mg/kg.
Data irritation
Skin-rabbit 500 mg Mild; Eyes-rabbit 100 mg/4 seconds Severe.
Flammability and hazard characteristics
It is non-combustible with fire releasing toxic fluoride and sodium oxide, silica smoke; when it is reacted with acid, it can generate toxic hydrogen fluoride.
Storage characteristics
Treasury: ventilation, low-temperature and drying; store it separately from food and acid.
Fire extinguishing agent
plenty of water.
Professional standards
TWA 2.5 mg (fluorine)/cubic meter.
Chemical Properties
Sodium hexafluorosilicate is a white crystalline solid.
Chemical Properties
white powder
Uses
In enamels for china and porcelain; manufacture of opal glass; as insecticide, rodenticide; mothproofing of woolens. Fluoridating agent for drinking water. Intermediate in production of synthetic cryolite.
Uses
Sodium hexafluorosilicate is used as a additive for water fluoridation, opal glass raw material and in ore refining. It is also used in the preparation of other fluoride chemicals like sodium fluoride, sodium hexafluoroaluminate, potassium hexafluorosilicate, ammonium fluoride and calcium fluoride. It finds application as flotator, component for wet etching, wood preservative, brazing flux and welding compound. It is an active component of concrete.
General Description
Fine, white, odorless, powdered solid. Toxic by ingestion, inhalation and skin absorption. Used as a rodenticide.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium fluorosilicate has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Hazard
Toxic by ingestion and inhalation, strong irritant to tissue.
Health Hazard
Inhalation of dust may irritate nose and throat. Ingestion causes symptoms similar to fluoride poisoning; compound is highly toxic; initial symptoms include nausea, cramps, vomiting, diarrhea, and dehydration; in severe cases, convulsions, shock, and cyanosis are followed by death in 2-4 hr. Contact with eyes causes irritation. Contact with skin causes rash, redness, and burning, sometimes followed by ulcer formation.
Fire Hazard
Behavior in Fire: Decomposes at red heat
Flammability and Explosibility
Non flammable
Industrial uses
Sodium fluorosilicate (Na2SiF6) is a white, isomorphous powder. Solubility in water is similar to that of NaF. In
acid pH, solubility improves.
Commercially, Na2SiF6 is produced by treatment of fluorosilic acid with sodium chloride,
as shown in the following reaction:
H2SiF6 + 2NaCl = Na2SiF6 + 2HCl
Safety Profile
Poison by ingestion and subcutaneous routes. A skin and severe eye irritant. An insecticide. When heated to decomposition it emits very toxic fumes of Fand Na2O.
Potential Exposure
Sodium hexafluorosilicate is as a rodenticide; as an intermediate in production of synthetic cyrolite; as an insecticide in delousing and in mothproofing of woolens.
Shipping
UN2674/Sodium fluorosilicate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise it from hot water (40mL/g) by cooling.
Incompatibilities
Reacts with acids to produce hydrogen fluoride, a highly corrosive and poisonous gas.
Sodium fluorosilicate Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5872 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32836 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
| Win-Win Chemical CO., Limited | 0086-577-64498589 | sales@win-winchemical.com | CHINA | 998 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Sodium fluorosilicate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-19 | Sodium fluorosilicate
16893-85-9
|
US $100.00-50.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2023-09-05 | sodium silicofluoride
16893-85-9
|
US $100.00 / KG | 1KG | 99% | 5000KG | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-06-17 | Sodium fluorosilicate
16893-85-9
|
US $100.00 / kg | 1kg | 99% | 500t/month | Henan Bao Enluo International TradeCo.,LTD |
-

- Sodium fluorosilicate
16893-85-9
- US $100.00-50.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- sodium silicofluoride
16893-85-9
- US $100.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Sodium fluorosilicate
16893-85-9
- US $100.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD