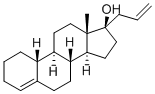
Allylestrenol
- CAS No.
- 432-60-0
- Chemical Name:
- Allylestrenol
- Synonyms
- organon;Turinal;gestanin;gestanol;gestanon;gestanyn;orageston;NSC 37723;ALLYLESTRENOL;allyl-estreno
- CBNumber:
- CB7739529
- Molecular Formula:
- C21H32O
- Molecular Weight:
- 300.48
- MDL Number:
- MFCD00198957
- MOL File:
- 432-60-0.mol
- MSDS File:
- SDS
| Melting point | 79.5-80° |
|---|---|
| Boiling point | 381.7°C (rough estimate) |
| Density | 0.9914 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 14.95±0.40(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| Merck | 14,291 |
| CAS DataBase Reference | 432-60-0(CAS DataBase Reference) |
| FDA UNII | I47VB5DZ8O |
| ATC code | G03DC01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| RTECS | KG7960000 |
Allylestrenol price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | A554755 | Allylestrenol | 432-60-0 | 100mg | $50 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FA55289 | Allylestrenol | 432-60-0 | 500mg | $60 | 2021-12-16 | Buy |
| Chem-Impex | 28191 | Allylestrenol,97-102%(assay) 97-102%(assay) | 432-60-0 | 250MG | $44.8 | 2021-12-16 | Buy |
| Chem-Impex | 28191 | Allylestrenol,97-102%(assay) 97-102%(assay) | 432-60-0 | 10G | $817.6 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 18468 | Allylestrenol | 432-60-0 | 5mg | $950 | 2021-12-16 | Buy |
Allylestrenol Chemical Properties,Uses,Production
Originator
Alilestrenol ,Terapia
Uses
A synthetic steroid with progestational activity.
Uses
Allylestrenol, is a synthetic sexualsteroid, that is used worldwide in case of endangered pregnancies.
Definition
ChEBI: Allylestrenol is a steroid. It derives from a hydride of an estrane.
Manufacturing Process
To 145 ml of dry methylamine which is cooled to -20°C 1.5 g of lithium cut to small pieces are added. To the solution which is blue in color after 10-20 min,
a solution of 3.0 g of oestradiol-3-methylether in 145 ml of absolute ether is
added drop wise. Subsequently the reaction mixture is stirred at -10°C for 40
h, after which 50 ml of absolute ethanol are added. Then the methylamine is
distilled off at law pressure.
To the remaining solution 50 ml of ether and 50 ml of water are added. The
water layer is separated and extracted with ether. The ethereal layer is
washed with a 2 N hydrochloric acid solution, subsequently with a saturated
sodium bicarbonate solution, and then with water. The ethereal solution is
dried and evaporated to dryness. The resulting crude reaction product is
dissolved in a mixture of benzene and petroleum ether (1:3) and
chromatographed over aluminium oxide. The δ4-17β-hydroxy-oestrene
obtained after chromatographic purification has a melting point of 80°-90°C
and 95°-100°C after repeated crystallization from petroleum ether.
A solution of 13.2 g of chromium trioxide in a mixture of 120 ml of water and
20 ml of acetic acid is added, with stirring, to a solution of 20 g of δ4-17β-
hydroxy-oestrene in 400 ml of benzene. Subsequently the reaction mixture is
vigorously stirred at room temperature for 16 h, after which the benzene layer
is separated.
The remaining aqueous layer is extracted a few times with benzene and the
benzene extracts collected are then added to the separated benzene layer.
The benzene extracts are successively washed with dilute sulfuric acid and
water and then evaporated to dryness. The residue is crystallized from
acetone, and the δ4-17β-oxo-oestrene, melting point 114°-116°C is obtained.
To a mixture of 22.4 ml of absolute ether and 1.84 g of magnesium, a mixture
of 2.72 ml of allyl bromide and 2.72 ml of absolute ether is added in nitrogen
atmosphere. Subsequently a solution of 2 g of δ4-17β-oxo-oestrene in 30 ml
of absolute ether is added to this reaction mixture, after which the whole is
stirred for 4 h. Then the reaction mixture is poured into acidified ice water.
The aqueous mixture is extracted with ether; the ether layer is separated,
washed with water, dried over sodium sulfate and evaporated to dryness. The
residue is recrystallized from a mixture of ether and petroleum ether, giving
δ4-17β-hydroxy-17α-allyl-oestrene, melting point 79.5°-80°C.
Therapeutic Function
Progestin; Antiandrogen
Allylestrenol Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 | sales@chembj.net | China | 269 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | fanfan@kangcang.com.cn | China | 340 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Allylestrenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-27 | Allylestrenol
432-60-0
|
US $0.00-0.00 / g | 1g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-26 | Allylestrenol
432-60-0
|
US $20.00-10.00 / kg | 1kg | 98% | 20 | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-26 | Allylestrenol
432-60-0
|
US $9.00-60.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD |
-

- Allylestrenol
432-60-0
- US $0.00-0.00 / g
- 99%
- Wuhan Cell Pharmaceutical Co., Ltd
-

- Allylestrenol
432-60-0
- US $20.00-10.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD
-

- Allylestrenol
432-60-0
- US $9.00-60.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD