Tizanidine
- CAS No.
- 51322-75-9
- Chemical Name:
- Tizanidine
- Synonyms
- AB-021;C07452;TERNELIN;SIRDALUD;ZANAFLEX;DS-103-282;TIZANIDINE;TIZANIDINE HCL;Tizanidine USP/EP/BP;TIZANIDINE HCL 2% GRANULES
- CBNumber:
- CB9670538
- Molecular Formula:
- C9H8ClN5S
- Molecular Weight:
- 253.71
- MDL Number:
- MFCD00865192
- MOL File:
- 51322-75-9.mol
| Melting point | 221-223° |
|---|---|
| Boiling point | 391.2±52.0 °C(Predicted) |
| Density | 1.82±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | H2O: ~29 mg/mL |
| form | solid |
| pka | 7.48±0.10(Predicted) |
| color | white |
| CAS DataBase Reference | 51322-75-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 6AI06C00GW |
| NCI Drug Dictionary | Zanaflex |
| ATC code | M03BX02 |
| NIST Chemistry Reference | Tizanidine(51322-75-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361-H302+H332 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | DK9910000 |
| Toxicity | LD50 orally in mice: 235 mg/kg (Sayers) |
Tizanidine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ApexBio Technology | B1063 | Tizanidine | 51322-75-9 | 50mg | $77 | 2021-12-16 | Buy |
| ApexBio Technology | B1063 | Tizanidine | 51322-75-9 | 500mg | $553 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0004432 | TIZANIDINE 95.00% | 51322-75-9 | 1G | $721.57 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0004432 | TIZANIDINE 95.00% | 51322-75-9 | 2.5G | $983.88 | 2021-12-16 | Buy |
| Crysdot | CD31001684 | Tizanidine 98+% | 51322-75-9 | 100mg | $77 | 2021-12-16 | Buy |
Tizanidine Chemical Properties,Uses,Production
Outline
Tizanidine is an imidazoline two nitrogen heterocyclic pentene derivative. The structure is similar to that of clonidine. In 1987, it was first listed in Finland as a central adrenalin α2 receptor agonist. Currently, it is used as a central muscle relaxant in clinic. It can be used to treat painful muscle spasm, such as neck waist syndrome and torticollis. It can also be used to treat postoperative pain, such as disc herniation and hip arthritis. It comes from the ankylosis of neurological disorders, such as multiple sclerosis, chronic myelopathy, cerebrovascular accident and etc.
Pharmacology
It selectively reduces the release of excitatory amino acids from interneurons and inhibits the multi synaptic mechanism related to muscle overstrain. This product does not affect the transmission of neuromuscular. It is well tolerated. It is effective for acute painful muscle spasms and chronic ankylosis originates from the spinal cord and brain. It can reduce the resistance of passive movement, reduce spasticity and clonus, and increase the intensity of voluntary movement.
Function
It is used to reduce skeletal muscle tension, muscle spasm and myotonia caused by brain and spinal cord injury, cerebral hemorrhage, encephalitis and multiple sclerosis.
Drug Interaction
- In vitro studies of human liver microsomal cytochrome P450 isozymes showed that Tizanidine and its metabolites do not affect the metabolism of other enzymes.
- Tizanidine makes the peak time of acetaminophen delayed by 16 minutes, but paracetamol does not affect the pharmacokinetic parameters of Tizanidine.
- Ethanol increases the area under the curve of Tizanidine by 20%, resulting in an increase of 15% in the maximum peak concentration and an increased side effect in Tizanidine.The central nervous system inhibition of ethanol and Tizanidine has additive effect.
- 4mg Tizanidine’s single or repeated retrospective study showed that the use of oral contraceptives compared with patients who did not take oral contraceptives at the same time reduced the scavenging rate of Tizanidine Hydrochloride by 50%.
- When combined with antihypertensive drugs and diuretics, hypotension and bradycardia can be induced.
- It is prohibited to use combined with fluoxamine or ciprofloxacin(Cytochrome oxidase CYP1A2 inhibitor). Clinical studies showed that the pharmacokinetic parameters of Tizanidine (area under the curve, elimination half-life, maximum blood concentration, and oral bioavailability) will be increased when combined with fluoxamine and ciprofloxacin simultaneously, while the plasma clearance rate will be decreased. This pharmacokinetic interaction may lead to serious adverse events.
Precaution
- The following patients should be cautious when taking the drug:
②Patients with renal insufficiency: It is reported that the drug is slowly excreted by kidney and easy to maintain high blood concentration.
- At the initial stage, its use may cause a sharp drop in blood pressure
- This medicine can cause reflex movement ability and sleepiness, so it is not suitable for driving or manipulating machinery during medication.
- There are differences in pharmacokinetics of different formulations of the drug. Food also plays a complex role in the pharmacokinetics of the drug.
Adverse reaction
For a small dose of painful muscle spasm, only mild transient lethargy, fatigue, dizziness, dry mouth, nausea and a slight decrease in blood pressure can be observed.
When used for spastic paralysis, the above adverse reactions are more common and obvious because of larger doses, but there is no need to stop taking drugs.
Contraindication
It is forbidden for those who are allergic to this medicine.
Description
Tizanidine is a centrally-acting muscle relaxant useful in the treatment of muscle spasms and a variety of spastic conditions.
Originator
Sandoz (Switzerland)
Uses
Tizanidine could have therapeutic use as a SARS-CoV-2 main protease inhibitor.
Uses
Labeled Tizanidine, intended for use as an internal standard for the quantification of Tizanidine by GC- or LC-mass spectrometry.
Definition
ChEBI: 2,1,3-Benzothiadiazole substituted at C-4 by a Delta1-imidazolin-2-ylamino group and at C-4 by a chloro group. It is an agonist at alpha2-adrenergic receptor sites.
Manufacturing Process
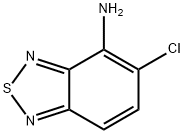
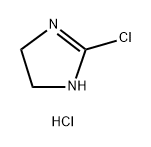
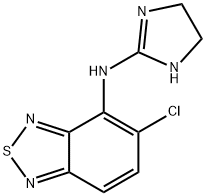
14 ml of benzoyl chloride are added to a solution of 11.5 g of ammonium thiocyanate in 150 ml of acetone in an ice bath and the mixture is then stirred for 10 min. This solution is heated to the boil at reflux together with 19 g of 4-amino-5-chloro-2,1,3-benzothiadiazole. The solution is cooled to room temperature and diluted with a 4-fold quantity of water. The precipitate is filtered off and rapidly brought to a boil together with 150 ml of a 2 N aqueous sodium hydroxide solution and kept at the boil for 5 min. The solution is cooled to room temperature, is acidified weakly with glacial acetic acid, the precipitate is filtered off, washed with ether and recrystallized from methanol. The N-(5-chloro-2,1,3-benzothiadiazol-4-yl)thiourea, obtained and this is boiled for 1 h together with 9 g of methyl iodide in 150 ml of methanol. After concentrating by evaporation, crude S-methyl-N-(5-chloro-2,1,3- benzothiadiazol-4-yl)isothiuronium iodide is obtained. 9.8 g of S-methyl-N-(5- chloro-2,1,3-benzothiadiazol-4-yl)isothiuronium iodide are heated to the boil at reflux for 1 h together with 50 ml of methanol and 1.8 ml of ethylene diamine. The solvent is then removed by evaporation and the moist residue is boiled at reflux for 1 h together with 20 ml of n-amyl alcohol. The mixture is subsequently shaken with 50 ml of chloroform and 150 ml of water until all the material is dissolved. 40 ml of a 2 N aqueous sodium hydroxide solution are added to the aqueous phase and extraction is effected with 200 ml of chloroform. The organic phase is dried and concentrated by evaporation. After recrystallizing the residue from methanol with the addition of some active charcoal, 4-(2-imidazolin-2-yl-amino)-5-chloro-2,1,3-benzothiadiazole, having a melting point of 221-223°C, is obtained. The 4-(2-imidazolin-2-yl-amino)-5-chloro-2,1,3-benzothiadiazole hydrochloride may be obtained by the teaction of 4-(2-imidazolin-2-yl-amino)-5-chloro- 2,1,3-benzothiadiazole with hydrochloric acid.
brand name
SIRDALUD
Therapeutic Function
Muscle relaxant, Spasmolytic
Mechanism of action
Tizanidine is a centrally active muscle relaxant analogue of clonidine that is approved for use in reducing spasticity associated with cerebral or spinal cord injury. Its mechanism of action for reducing spasticity suggests presynaptic inhibition of motor neurons at the α2-adrenergic receptor sites, reducing the release of excitatory amino acids and inhibiting facilitatory ceruleospinal pathways, thus resulting in a reduction in spasticity. Tizanidine only has a small fraction of the antihypertensive action of clonidine, presumably because of action at a selective subgroup of α2C-adrenoceptors, which appear to be responsible for the analgesic and antispasmodic activity of imidazoline α2-agonists(20).
Clinical Use
Tizanidine is a centrally acting adrenergic α2 receptor agonist used to treat chronic muscle spasticity conditions, such as multiple sclerosis.
Side effects
Postulated mechanisms include α2 receptor-mediated decreased release of norepinephrine and serotonin from spinal interneurons. Tizanidine is structurally related to the α2 agonist clonidine that is used to treat hypertension; however, the blood pressure–lowering potency of tizanidine is approximately 10 to 20% that of clonidine. Nevertheless, patients may experience hypotension with tizanidine, together with muscle weakness, that may result in dizziness and falls in mobile patients. Tizanidine is rapidly and almost completely absorbed from the gastrointestinal tract; however, the estimated bioavailability is only 10 to 15% because of extensive first-pass metabolism, mainly by CYP1A2, which results in oxidative degradation of the imidazoline ring and hydroxylation of the aromatic system. Elevated liver enzyme values are not frequent with tizanidine use. Hepatic injury and death because of liver failure have been reported, however, and this complication should be considered in view of its marginal antispasmodic efficacy. Other frequently reported side effects of tizanidine are drowsiness and dry mouth. Clonidine also has been used to treat spasticity; however, even less high-quality clinical study data are available for this agent.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: enhanced muscle relaxant effect
with procainamide.
Antibacterials: concentration increased by
ciprofloxacin - avoid; concentration possibly
increased by norfloxacin; concentration possibly
reduced by rifampicin.
Antidepressants: concentration increased by
fluvoxamine - avoid.
Antihypertensives: enhanced hypotensive effect.
Oral contraceptives: clearance of tizanidine reduced
by 50
%.
Metabolism
Tizanidine undergoes rapid and extensive first pass metabolism in the liver mainly via the cytochrome P450 isoenzyme CYP1A2. The metabolites (mainly inactive) constitute 70
% of the administered dose and are excreted via the renal route.
Tizanidine Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3581 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9271 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29067 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD | +8617731987558 | xingjiu@xingjiubiotech.com | China | 990 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 | dj@sgtlifesciences.com | China | 12382 | 58 |
| Coresyn Pharmatech Co., Ltd. | +86-571-86626709 +86-18157142896 | cbc@coresyn.com | China | 9991 | 58 |
View Lastest Price from Tizanidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-03-27 | Tizanidine
51322-75-9
|
US $50.00 / KG | 1KG | 99.9% | 500MT/month | XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD | |
 |
2021-08-04 | Tizanidine USP/EP/BP
51322-75-9
|
US $1.10 / g | 1g | 0.999 | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-20 | Tizanidine
51322-75-9
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd |
-

- Tizanidine
51322-75-9
- US $50.00 / KG
- 99.9%
- XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD
-

- Tizanidine USP/EP/BP
51322-75-9
- US $1.10 / g
- 0.999
- Dideu Industries Group Limited
-

- Tizanidine
51322-75-9
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd
51322-75-9(Tizanidine)Related Search:
1of4





