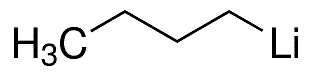
n-Butyllithium
- CAS No.
- 109-72-8
- Chemical Name:
- n-Butyllithium
- Synonyms
- N-BULI;BUTYLLITHIUM;Lithiumn-butyl;n-ButyllithiuM(BULI);ButyllithiuminhexaneM;n-Butyllithium, 1.6M solution in hexanes;butyl-lithiu;LITHIUM-1-BUTANIDE;Butyllithium solution;N-butyl lithium Solution
- CBNumber:
- CB9702303
- Molecular Formula:
- C4H9Li
- Molecular Weight:
- 64.06
- MDL Number:
- MFCD00009414
- MOL File:
- 109-72-8.mol
- MSDS File:
- SDS
| Melting point | -95 °C |
|---|---|
| Boiling point | 80 °C |
| Density | 0.68 g/mL at 20 °C |
| Flash point | 10 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with diethyl ether and cyclohexane. |
| form | liquid |
| color | yellow |
| Specific Gravity | 0.695 |
| Odor | Odor of the solvent |
| Water Solubility | vigorous reaction |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1209227 |
| Exposure limits |
ACGIH: TWA 50 ppm (Skin) OSHA: TWA 500 ppm(1800 mg/m3) NIOSH: IDLH 1100 ppm; TWA 50 ppm(180 mg/m3) |
| LogP | 1.25-2.31 |
| Indirect Additives used in Food Contact Substances | BUTYLLITHIUM |
| CAS DataBase Reference | 109-72-8(CAS DataBase Reference) |
| FDA UNII | 09W9A6B8ZC |
| NIST Chemistry Reference | Butyl lithium(109-72-8) |
| EPA Substance Registry System | Lithium, butyl- (109-72-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS02,GHS05,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H250-H261-H304-H314-H336-H361f-H373-H411-H260-H361d-H410-H252-H318-H332-H401-H361 | |||||||||
| Precautionary statements | P210-P222-P231+P232-P261-P273-P422-P223-P370+P378-P280-P301+P310-P302+P334-P303+P361+P353-P304+P340+P310-P305+P351+P338-P331-P201-P301+P310a-P405-P422a-P501a-P262 | |||||||||
| Hazard Codes | F,C,N | |||||||||
| Risk Statements | 14/15-17-34-48/20-51/53-62-65-67-63-35-11-15-50/53-66 | |||||||||
| Safety Statements | 6-9-16-26-36/37/39-45-61-62-6A-46-43B-43-60-33-29-5 | |||||||||
| RIDADR | UN 3399 4.3/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| Toxicity | There is little toxicity data available for the butyllithiums; for data on ether and hydrocarbon solvents, see the appropriate LCSSs. | |||||||||
| NFPA 704 |
|
n-Butyllithium price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 230707 | n-Butyllithium solution 2.5?M in hexanes | 109-72-8 | 18L | $2110 | 2024-03-01 | Buy |
| Sigma-Aldrich | 20159 | n-Butyllithium solution 2.7?M in heptane | 109-72-8 | 100mL | $93.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 20159 | n-Butyllithium solution 2.7?M in heptane | 109-72-8 | 500mL | $224 | 2024-03-01 | Buy |
| Sigma-Aldrich | 186171 | n-Butyllithium solution 1.6M in hexanes | 109-72-8 | 8l | $941 | 2024-03-01 | Buy |
| Sigma-Aldrich | 186171 | n-Butyllithium solution 1.6?M in hexanes | 109-72-8 | 18L | $1680 | 2024-03-01 | Buy |
n-Butyllithium Chemical Properties,Uses,Production
Chemical Properties
n-Butyllithium is a Colorless or slightly yellow transparent liquid at room temperature, also comes in powder form. Boiling point is 80-90℃(0.013Pa), relative density is 0.68-0.70. Soluble in many organic solvents. Stable in room temperature, lithium hydride removable by heating. Disintegrates in water, soluble in hydrocarbons and ethers, forms complex compounds with ethers, amines, and sulfides, and uses N-hexane as a solvent.

Uses
N-Butyllithium solution is mostly used as a pharmaceutical intermediate to synthesize anionic polymerization initiator. It is also used to produce rubber (such as integral rubber SIBR, which is the most versatile diene rubber to date), fragrance, liquid crystal materials, and other industries. It is a chemical product intermediate, linking agent, alkylating agent, and polymerization catalyst. As a catalyst, N-Butyllithium is widely used as a pharmaceutical intermediate, liquid crystal monomer, and pesticide production catalyst.
N-Butyllithium is also widely used in organic syntheses, especially in growing carbon chains, and it is a staple laboratory product in reactions such as:
1) Metallization reaction: R-H + n-Butyl-Li → R-Li + Butane, in which the produced lithium alkyl can react with many substances.
2) Direct metallization: the aromatic compound that connects the substitute reacts with N-Butyllithium, and lithium metal can be attached to the aromatic compound.
3) Nucleophilic addition and substitution reaction.
4) Halogen-metal replacement.
Identifying Procedures
N-Butyllithium must be calibrated through single titration as follows:
1) Titration solution: 1mol/L SEC butanol/dimethylbenzene solution (butanol and dimethylbenzene must be dried with an activated 5A molecular sieve)
2) Indicator: 2,2’-dipyridine
3) Solvent: dimethylbenzene (must be dried with an activated 5A molecular sieve).
4) Operation method: Under the protection of argon, add magneton, 20ml dimethylbenzene and a small amount of indicator into a 100ml 3-lipped bottle with a tipping plug. Then use a precisely marked 2ml injector to swiftly transfer 2ml N-Butyllithium into the bottle (the air in the injector must be replaced with argon, which must then be expelled when collecting the N-Butyllithium; fill and drain the injector in the N-Butyllithium multiple times to prevent any water or air from remaining in the injector). The system should turn a purplish red. Then, wash, dry, and rinse the same injector with titration solution 2-3 times (to prevent any change in the amount added) and titrate the solution until the system turns yellow, and cease titration.
5) Repeat titration once; if the percent error between the two times is within 2%, the result can be regarded as correct.
6) Titration result: titration solution (ml)/2 to obtain N-Butyllithium concentration.
Warnings and precautions
1) N-Butyllithium is extremely combustible when in contact with air; when measuring, the needle of the injector will eject sparks.
2) The entire process must be protected by argon for safety precaution.
3) If N-Butyllithium catches fire, it must be extinguished with sand, which must be placed within arm’s reach at all times.
When preparing and using N-Butyllithium, do not operate alone in case of emergency
Category
Spontaneously combustible when in contact with water
Explosivity characteristics
May explode in combination with phenylethylene
Flammability characertistics
Combusts in air at a concentration abover 20%; combusts upon contact with water and carbon dioxide; flammable when in contact with heat and open flame.
Storage
Store in ventilated, cool and dry spaces; must be waterproof and carbon dioxide-proof.
Extinguisher
Dry powder
Chemical Properties
n-Butyllithium is a colorless crystalline solid or clear yellow solution. Due to its high reactivity and extreme solubility, even in its impurities, it is commonly thought to be a viscous liquid.
Uses
n-Butyllithium is a strong nucleophile in the synthetic organic chemistry. It is also used as an initiator in the polymerization process.
Uses
n-Butyllithium is used as a polymerization initiator in the production of elastomers like polybutadiene and styrene- butadiene- styrene. As a strong base, it is utilized in the synthesis of organic compounds. It undergoes thermal decomposition to prepare 1-butene.
Preparation
Industrially, n-butyllithium is produced by the
reaction of n-butyl chloride with lithium metal dispersion in various hydrocarbon solvents.
Hexane is the most commonly used solvent. Up to one-half of the lithium is replaced with
sodium in order to lower the cost of the butyllithium and increase the reactivity of the dispersion.
The reaction is carried out below the boiling point of the solvent.
The laboratory procedure is essentially the same except that pentane and diethyl ether
are two of the more popular solvents. The preparation runs smoothly in ether at lower
temperatures but the product must either be used immediately or kept refrigerated due to
the rapid cleavage of ether by n-butyllithium.
Definition
ChEBI: Butyllithium is an alkyllithium compound.
Flammability and Explosibility
The risk of fire or explosion on exposure of butyllithium solutions to the atmosphere depends on the identity of the organolithium compound, the nature of the solvent, the concentration of the solution, and the humidity. t-Butyllithium solutions are the most pyrophoric and may ignite spontaneously on exposure to air. Dilute solutions (1.6 M, 15% or less) of n-butyllithium in hydrocarbon solvents, although highly flammable, have a low degree of pyrophoricity and do not spontaneously ignite. Under normal laboratory conditions (25 °C, relative humidity of 70% or less), solutions of -20% concentration will usually not ignite spontaneously on exposure to air. More concentrated solutions of n-butyllithium (50 to 80%) are most dangerous and will immediately ignite on exposure to air. Contact with water or moist materials can lead to fires and explosions, and the butyllithiums also react violently with oxygen.
storage
In particular, butyllithium should be stored and handled in areas free of ignition sources, and containers of butyllithium should be stored under an inert atmosphere. Work with butyllithium should be conducted in a fume hood under an inert gas such as nitrogen or argon. Safety glasses, impermeable gloves, and a fire-retardant laboratory coat are required.
Incompatibilities
The butyllithiums are extremely reactive organometallic compounds. Violent explosions occur on contact with water with ignition of the solvent and of the butane produced. t-Butyllithium will ignite spontaneously in air. The butyllithiums ignite on contact with water, carbon dioxide, and halogenated hydrocarbons. The butyllithiums are incompatible with acids, halogenated hydrocarbons, alcohols, and many other classes of organic compounds.
Waste Disposal
Excess butyllithium solution can be destroyed by dilution with hydrocarbon solvent to a concentration of
approximately 5 wt %, followed by gradual addition to water with vigorous stirring under an inert
atmosphere. Alternatively, the butyllithium solution can be slowly poured (transfer by cannula for s- or tbutyllithium)
into a plastic tub or other container of powdered dry ice.
The residues from the above procedures and excess butyllithium should be placed in an appropriate
container, clearly labeled, and handled according to your institution's waste disposal guidelines.
n-Butyllithium Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7933 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
Related articles
- Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n-Butyllithium
- n-Butyllithium is an organolithium reagent, a good nucleophile and a strong base (pKa ~ 48). n-Butyllithium is a good nucleoph....
- Apr 22,2024
- n-Butyllithium: A Comprehensive Review of Properties, Praparation and Reactions
- The chemical compound n-butyllithium (abbreviated BuLi) is the most prominent organolithium reagent.
- May 11,2023
- n-Butyllithium-Hazard and Toxicity
- n-Butyllithium (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the produc....
- Sep 6,2019
View Lastest Price from n-Butyllithium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-23 | n-Butyllithium
109-72-8
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-11-16 | n-Butyllithium
109-72-8
|
US $0.00-0.00 / kg | 1kg | 99% | 50000kg | Hebei Kingfiner Technology Development Co.Ltd | |
 |
2023-09-21 | n-Butyllithium
109-72-8
|
US $36.83 / KG | 1KG | 99% | 15MT | Wuhan Han Sheng New Material Technology Co.,Ltd |
-

- n-Butyllithium
109-72-8
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- n-Butyllithium
109-72-8
- US $0.00-0.00 / kg
- 99%
- Hebei Kingfiner Technology Development Co.Ltd
-

- n-Butyllithium
109-72-8
- US $36.83 / KG
- 99%
- Wuhan Han Sheng New Material Technology Co.,Ltd