- β-Propiolactone
-

- $10.00/ kg
-
2024-04-22
- CAS:57-57-8
- Min. Order: 1kg
- Purity: 99.7%
- Supply Ability: 200000kg
- 2-Oxetanone
-
- $8.00 / 1KG
-
2024-04-09
- CAS:57-57-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 2-Oxetanone
-

- $0.00 / 1kg
-
2023-12-04
- CAS:57-57-8
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 5000kg
Related articles - Uses of Beta-propiolactone
- Beta-propiolactone is a colorless liquid with a strong, slightly sweet odor. It may occur naturally, but no clear documentatio....
- Nov 24,2021
|
| | 2-Oxetanone Basic information |
| | 2-Oxetanone Chemical Properties |
| Melting point | -33 °C (lit.) | | Boiling point | 162 °C (lit.) | | density | 1.146 g/mL at 25 °C (lit.) | | vapor pressure | 3 at 25 °C (NIOSH, 1997) | | refractive index | n20/D 1.412(lit.) | | Fp | 158 °F | | storage temp. | -20°C | | solubility | Miscible with acetone, alcohol, chloroform, and ether (Windholz et al., 1983) | | form | Liquid | | color | Colorless liquid with a sweet but irritating odor | | Odor | pungent odor | | Water Solubility | 37 g/100 mL | | Sensitive | Moisture Sensitive | | Merck | 14,7820 | | Henry's Law Constant | 7.6 at 25 °C (approximate - calculated from water solubility and vapor pressure) | | Stability: | Moisture Sensitive, Very Hygroscopic | | InChIKey | VEZXCJBBBCKRPI-UHFFFAOYSA-N | | CAS DataBase Reference | 57-57-8(CAS DataBase Reference) | | IARC | 2B (Vol. 4, Sup 7, 71) 1999 | | NIST Chemistry Reference | «beta»-Propiolactone(57-57-8) | | EPA Substance Registry System | beta-Propiolactone (57-57-8) |
| Hazard Codes | T+ | | Risk Statements | 45-26-36/38 | | Safety Statements | 53-45-99 | | RIDADR | UN 3382 6.1/PG 1 | | WGK Germany | 3 | | RTECS | RQ7350000 | | TSCA | Yes | | HazardClass | 6.1 | | PackingGroup | I | | HS Code | 29322090 | | Hazardous Substances Data | 57-57-8(Hazardous Substances Data) | | Toxicity | LC50 (inhalation) for rats 25 ppm/6-h (quoted, RTECS, 1985). |
| | 2-Oxetanone Usage And Synthesis |
| Description | Beta-propiolactone is a colorless liquid with a strong, slightly
sweet odor. It may occur naturally, but no clear documentation
of its occurrence in nature was found, and it must be synthesized
for commercial purposes. Beta-propiolactone is unstable
at room temperature but stable when stored at 5 ℃ in glass
containers.
Its tendency to be unstable and react with other molecules in
the vicinity is responsible for both its toxicity and its usefulness.
Significant commercial production of beta-propiolactone took
place during the late 1950s through the mid-1970s, when it was
widely used in chemical synthesis in reactions with other
molecules to produce new chemicals. All lactones are characterized
by a ring structure consisting of two or more carbon
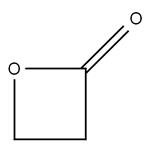
atoms – as can be seen from its structure, beta-propiolactone
has three in its ring – and a single oxygen, coupled with an
adjacent ketone. The fewer the carbons in the ring, the more
‘strained’ is the ring structure and the more unstable and reactive
its characteristics. When the ring bonds break, the betapropiolactone
molecules attach to other nearby molecules. | | Chemical Properties | β-Propiolactone is a colorless liquid which slowly hydrolyzes to hydracrylic acid and must be cooled to remain stable. It reacts with alcohols, acid chlorides, ammonia, and water to yield β-substituted propionic acid derivatives. The most important characteristic of the substance is its ability to polymerize. This highly exothermic process occurs simply by warming, although it is also catalyzed by both acid and base. | | Physical properties | β-Propiolactone is a colorless, highly reactive liquid,thatis solublein water, alcohol, acetone, and chloroform (solubilityinwaterat 25℃, 37 vol %). | | Uses | As late as 1974, b-propiolactone was used in the United States in the preparation of acrylic acid and acrylate esters. Today, its principal significance is as a reactive intermediate in organic syntheses; a small amount is treated with ammonia to provide balanine. b-Propiolactone was also used as a disinfectant. It appeared to be an attractive replacement for formaldehyde due to its 25-fold greater disinfecting power, but it has since been abandoned because of its carcinogenic properties. | | Uses | Versatile intermediate in organic synthesis. | | Uses | Reacts with bacteriphage DNA causing inactivation, repair and recombination | | Definition | ChEBI: Beta-propiolactone is a propan-3-olide. It is functionally related to a 3-hydroxypropionic acid. It derives from a hydride of an oxetane. | | Preparation | β-Propiolactone is synthesized
by passing equimolar amounts of ketene and
formaldehyde into either acetone or b-propiolactone itself. The reaction is carried out at low
temperature (<20℃) with a yield of ca. 90 %. Both aluminum chloride and zinc chloride
have been employed as catalysts, and the use of
methyl borate has also been suggested. | | Brand name | Betaprone (Forest). | | General Description | A colorless liquid with a slightly sweetish, pungent odor. Used as an intermediate in organic synthesis; disinfectant, sterilant for blood plasma, tissue grafts, vaccines, enzymes and surgical instruments. | | Air & Water Reactions | Slow reaction with water to form beta- hydroxypropionic acid. | | Reactivity Profile | 2-Oxetanone is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. 2-Oxetanone may be incompatible with alkalis. | | Hazard | Strong skin and upper respiratory tract irri-
tant, skin cancer. Possible carcinogen. Worker expo-
sure should be minimized. | | Health Hazard | The toxicity potential of 2-Oxetanone via inhalation or ingestion is high; may cause death or permanent injury after very short exposures to small quantities. It is a carcinogen. | | Fire Hazard | Containers may explode. When heated to decomposition, 2-Oxetanone emits acrid smoke and fumes. Stable when stored at 41F. Avoid storing in areas of exposure to the direct rays of the sun and in areas of high fire hazard. Tends to polymerize on storage. Avoid elevated temperatures. | | Safety Profile | Confirmed carcinogen
with experimental carcinogenic,
neoplastigenic, and tumorigenic data. Poison
by inhalation. Moderately toxic by
intraperitoneal route. An initiator. Human mutation data reported. When heated to
decomposition it emits acrid smoke and
irritating fumes. | | Potential Exposure | β-Propiolactone is used as a chemical
intermediate in synthesis of acrylic acid and esters, acrylate
plastics; as a vapor sterilizing agent; phase disinfectant;
and a viricidal agent. | | Carcinogenicity | β-Propiolactone is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals. | | Environmental fate | Chemical/Physical. Slowly hydrolyzes to hydracrylic acid (Windholz et al., 1983). In a reactor
heated to 250 °C and a pressure of 12 mmHg, β-propiolactone decomposed to give equal amounts
of ethylene and carbon dioxide (James and Wellington, 1969). | | Shipping | UN3382 Toxic by inhalation liquid, n.o.s. with
an LC 50 ≤1000 mL/m 3 and saturated vapor concentration
≥10 LC 50 , Hazard class: 6.1; Labels: 6.1 Technical
Name Required, Inhalation Hazard Zone B. UN2810 Toxic
liquids, organic, n.o.s., Hazard Class: 6.1; Labels:
6.1-Poisonous materials, Technical Name Required. | | Purification Methods | Fractionally distil the lactone from sodium under reduced pressure. It gives an acidic solution in H2O. It irritates the skin and is a possib | | Toxicity evaluation | It is soluble in water (370 g l�1 at 25°C) and miscible in other
common organic solvents including acetone, chloroform,
diethyl ether, and ethanol (Log Kow 0.462). Hydrolysis occurs
in water where the half-life in aqueous media at 25°C is
approximately 3.5 h. If released to soil, relatively rapid
hydrolysis can be expected to occur in the presence of moisture.
Significant evaporation may occur from dry surfaces. With
a vapor pressure of 3.4 mm Hg at 25°C, it can also vaporize
into the air as temperature rises. If released to the atmosphere,
beta-propiolactone is expected to exist in the gas phase, where
it may be relatively more persistent in the absence of moisture
than it is in aqueous media. The half-life for the reaction with
photochemically produced hydroxyl radicals was estimated to
be a relatively slow rate of 45 days in the atmosphere. | | Incompatibilities | Reacts with water, causing decomposi-
tion and forming 3-hydroxypropionic acid (CAS: 503-66-
3), an irritant. Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Incompatible with acet-
ates, halogens, thiocyanates, thiosulfates, strong oxidizers;
strong bases. Forms explosive mixture with air above 75℃.
May polymerize upon storage or due to warming. Stable if
kept under refrigeration @ 5 to 10 ℃/40 to 50 ℃. | | Waste Disposal | Dissolve or mix the material
with a combustible solvent and burn in a chemical incinera-
tor equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. |
| | 2-Oxetanone Preparation Products And Raw materials |
|