- Isovaleraldehyde
-

- $5.00 / 25kg
-
2024-04-30
- CAS:590-86-3
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 200ton/year
- Isovaleraldehyde
-

- $40.00 / 1kg
-
2023-10-17
- CAS:590-86-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Isovaleraldehyde
-

- $2300.00 / 1kg
-
2023-08-01
- CAS:590-86-3
- Min. Order: 25kg
- Purity: 99%
- Supply Ability: 20tons
|
| | Isovaleraldehyde Basic information |
| Product Name: | Isovaleraldehyde | | Synonyms: | ISOVALERALDEHYDE 97+%;ISOVALERALDEHYDE 95+% NATURAL;ISOVALERALDEHYDE(SG);Isoamylaldehyd;iso-C4H9CHO;Isovaleraldehyd;Isovalerylaldehyde;ISOVALERALDEHYDE, NATURAL | | CAS: | 590-86-3 | | MF: | C5H10O | | MW: | 86.13 | | EINECS: | 209-691-5 | | Product Categories: | | | Mol File: | 590-86-3.mol | 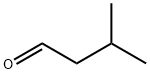 |
| | Isovaleraldehyde Chemical Properties |
| Melting point | -60 °C | | Boiling point | 90 °C (lit.) | | density | 0.803 g/mL at 25 °C (lit.) | | vapor density | 2.96 (vs air) | | vapor pressure | 30 mm Hg ( 20 °C) | | refractive index | n20/D 1.388(lit.) | | FEMA | 2692 | 3-METHYLBUTYRALDEHYDE | | Fp | 29 °F | | storage temp. | 2-8°C | | solubility | alcohol: miscible | | form | Liquid | | color | Clear colorless to light yellow | | Odor | at 0.10 % in dipropylene glycol. ethereal aldehydic chocolate peach fatty | | Odor Type | aldehydic | | explosive limit | 1.7-6.8%(V) | | Odor Threshold | 0.0001ppm | | Water Solubility | 15 g/L (20 ºC) | | Merck | 14,5229 | | JECFA Number | 258 | | BRN | 773692 | | Stability: | Stable, but light and air sensitive. Highly flammable. Readily forms explosive mixtures with air. Incompatible with strong oxidizing agents, bases, reducing agents, air. | | InChIKey | YGHRJJRRZDOVPD-UHFFFAOYSA-N | | LogP | 1.5 at 25℃ and pH7 | | CAS DataBase Reference | 590-86-3(CAS DataBase Reference) | | NIST Chemistry Reference | Butanal, 3-methyl-(590-86-3) | | EPA Substance Registry System | Isovaleraldehyde (590-86-3) |
| | Isovaleraldehyde Usage And Synthesis |
| Chemical Properties | 3-Methylbutyraldehyde has a choking, powerful, acrid, pungent, apple-like odor. This compound is also reported to
have a fruity, fatty, animal, almond odor. | | Chemical Properties | Isovaleraldehyde is a colorless, low-solubility liquid with a
pungent odor similar to that of apples. | | Occurrence | Reported found in over 180 natural sources including apple, banana, berries, grapes, peach, papaya,
peach, kohlrabi, carrot, celery, leek, peas, potato, bell pepper, tomato, ginger, peppermint and spearmint oil, other Mentha oils,
vinegar, breads, many cheeses, butter, milk, egg, fatty and lean fish, meats, hop oil, beer, cognac, sherry, rum, grape wines,
cocoa, coffee, tea, filberts, peanuts, pecans, peanut butter, barley, oats, soybean, honey, avocado, mace, plum, beans, mush�rooms, starfruit, mango, beetroot, cardamom, coriander seed, rice, lovage leaf, pumpkin, buckwheat, laurel, malt, clary sage,
wort, elderberry, clam, scallops, crab, crayfish, okra, sapodilla, truffles, kiwifruit and other sources. | | Uses | Isovaleraldehyde is manufactured by oxidizing isoamyl alcohol with sodium
perchromate and sulfuric acid. Isovaleraldehyde is present in
essential oils of orange, peppermint, lemon, and other plants
and fruits. Its main uses are as an artificial flavor additive and
in perfumes. | | Uses | Isovaleraldehyde acts as a reagent in the preparation of active pharmaceutical ingredient (API) products. It serves as an internal standard for the determination of wine aroma carbonyl compounds with 5-8 carbon atoms. Further, it is utilized as a standard to evaluate the quality of olive oils by headspace solid-phase microextraction-gas chromatography using flame ionization detection and multivariate analysis. In addition to this, it is employed to enhance the taste and odor of the food. | | Uses | In artificial flavors and perfumes. | | Definition | ChEBI: 3-methylbutanal is a methylbutanal that is butanal substituted by a methyl group at position 3. It occurs as a volatile constituent in olives. It has a role as a flavouring agent, a plant metabolite, a volatile oil component and a Saccharomyces cerevisiae metabolite. | | Preparation | By oxidation of isoamyl alcohol. | | General Description | Colorless liquid with a weak suffocating odor. Floats on water. Produces an irritating vapor. It is a metabolite found in or produced by Saccharomyces cerevisiae. | | Air & Water Reactions | Highly flammable. | | Reactivity Profile | Isovaleraldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. | | Health Hazard | Inhalation causes chest discomfort, nausea, vomiting, and headache. Contact of liquid with eyes or skin causes irritation. Ingestion causes irritation of mouth and stomach. |
| | Isovaleraldehyde Preparation Products And Raw materials |
|