- Ertapenem
-

- $0.00 / 1kg
-
2024-04-29
- CAS:153832-46-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- Ertapenem
-
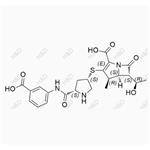
- $0.00 / 10mg
-
2024-04-27
- CAS:153832-46-3
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 10g
- Ertapenem
-

- $200.00 / 1kg
-
2023-06-26
- CAS:153832-46-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Month
|
| Product Name: | Ertapenem | | Synonyms: | ERTAPENEM;(4r,5r,6s)-3-[(3s,5s)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;ERTAPENEM(FORR&DONLY);(4R,5S,6S)-3-{[(3S,5S)-5-[(3-carboxyphenyl)carbaMoyl]pyrrolidin-3-yl]sulfanyl}-6-[(1R)-1-hydroxyethyl]-4-Methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid;Ertapenem hydrochloride;1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylicacid,3-[[(3S,5S)-5-[[(3-carboxyphenyl)aMino]carbonyl]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-Methyl-7-oxo-,(4R,5S,6S)-;sodium(4R,5S,6S)-3-(((3S,5S)-5-((3-carboxylatophenyl)carbamoyl)pyrrolidin-1-ium-3-yl)thio)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate;EOS-61119 | | CAS: | 153832-46-3 | | MF: | C22H25N3O7S | | MW: | 475.51 | | EINECS: | 1533716-785-6 | | Product Categories: | pharmaceutical intermediates | | Mol File: | 153832-46-3.mol | 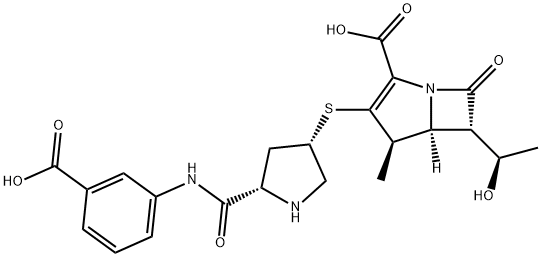 |
| | Ertapenem Chemical Properties |
| Boiling point | 813.9±65.0 °C(Predicted) | | density | 1.55±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | soluble in DMSO, Methanol | | pka | 4.03±0.10(Predicted) | | form | Foam | | color | Brown | | CAS DataBase Reference | 153832-46-3 |
| | Ertapenem Usage And Synthesis |
| Brand Name(s) in US | Invanz
| | Description | Ertapenem is another synthetic carbapenem with a rather complex side chain at C-3. It is used once daily
parenterally, with special application against anaerobes. As with meropenem, the 4-β-methyl group confers
stability toward dehydropeptidase-1 It is not active against pseudomonads or acinetobacteria and, therefore,
should not be substituted for imipenem or meropenem. It is relatively strongly bound to serum proteins, so it
has a prolonged half-life, making it more convenient to use than the other carbapenems when its spectrum
warrants this. Its reported indications include complicated intra-abdominal and complicated skin/skin
structure infections caused by sensitive organisms (for intra-abdominal: Escheri chia coli, Clostri di um
clostri doforme, Bacteroi des fragilis, and Peptostreptococcus sp; for skin/skin structures: Staphylococcus
aureus (methicillin-susceptible strains), Streptococcus pyogenes, E.coli, or Peptostreptococcus sp.). It can
be administered once daily. | | Uses | Antibacterial. Invanz (Merck). | | Uses | Ertapenem is a long-acting parenteral cabapenem. Ertapenem has bactericidal activity against a variety of gram-negative pathogens, some gram positive strains, and Haemopilus influenzae. Ertapenem has shown to inactivate L,D-transpeptidase, an enzyme responsible for in vitro cross-linking of Mycobacterium tuberculosis peptidoglycan. | | Definition | ChEBI: Meropenem in which the one of the two methyl groups attached to the amide nitrogen is replaced by hydrogen while the other is replaced by a 3-carboxyphenyl group. The sodium salt is used for the treatment of moderate to severe susceptible infections includ
ng intra-abdominal and acute gynaecological infections, pneumonia, and infections of the skin and of the urinary tract. | | Antimicrobial activity | Activity against aerobic and anaerobic pathogens is comparable
to that of imipenem: MIC values for Gramnegative
bacilli (with the exception of Ps. aeruginosa) are
generally lower and those for Gram-positive cocci higher.
Ertapenem is stable to most serine β-lactamases, but
is hydrolyzed by serine carbapenemases and metallo-
β-lactamases. | | General Description | Ertapenem (Invanz, for injection) is a synthetic 1-β-methylcarbapenem that is structurally related to β-lactam antibiotics,particularly the thienamycin group. Its mechanism ofaction is the same as that of other β-lactam antibiotics. Thestructure resists β-lactamases and dehydropeptidases.
Ertapenem is indicated for the treatment of moderate tosevere infections caused by susceptible strains causing complicatedintra-abdominal infections such as Escherichia,Clostridium, Peptostreptococcus, and Bacteroides. Theantibiotic is also indicated for complicated skin and skinstructure infections including diabetic foot infections (withoutosteomyelitis). Treatable strains include Staphylococcus(MSSA), Streptococcus, Escherichia, Klebsiella, Proteus,and Bacteroides. Ertapenem is also indicated for community-acquired pneumonia caused by S. pneumoniae,Haemophilus infljuenzae, and M. catarrhalis. Complicatedurinary tract infections and acute pelvic infections round outthe indications for ertapenem. | | Pharmacokinetics | Cmax 1 g intramuscular: c. 67 mg/L after 2 h
1 g intravenous infusion (30 min): c. 155 mg/L end infusion
Plasma half-life: c. 4 h
Volume of distribution: c. 0.12 L/kg (steady state)
Plasma protein binding: 85–95%
Absorption after intramuscular injection is essentially complete
with 90% bioavailability. The modestly extended plasma
half-life allows once-daily dosing.
Excretion is predominantly by the renal route, about 80%
being recovered in the urine within 24 h. About 40% is eliminated
unchanged, the rest as a biologically inactive ringopened
metabolite. Dosage should be reduced in severe renal
impairment. | | Clinical Use | Complicated intra-abdominal infections
Complicated skin and skin structure infections, including diabetic foot
infections without osteomyelitis
Community acquired pneumonia
Complicated urinary tract infections including pyelonephritis
Acute pelvic infections including postpartum endomyometritis, septic
abortion and postsurgical gynecologic infections
Prophylaxis of surgical site infection following elective colorectal surgery | | Side effects | Ertapenem appears to be generally well tolerated. The most
common drug-related adverse experiences are diarrhea (5.5%),
infused vein complication (3.7%), nausea (3.1%), headache
(2.2%), vaginitis (2.1%), phlebitis/thrombophlebitis (1.3%)
and vomiting (1.1%). Seizures have occasionally been reported
(0.5%) in patients with a history of disorders of the CNS. | | Drug interactions | Potentially hazardous interactions with other drugs
Antiepileptics: concentration of valproate reduced -
avoid concomitant use | | Metabolism | After intravenous infusion of radiolabelled 1 g ertapenem,
the plasma radioactivity consists predominantly (94%)
of ertapenem. The major metabolite of ertapenem is the
ring-opened derivative formed by dehydropeptidase�I-mediated hydrolysis of the beta-lactam ring. Approximately 80% of a dose is recovered in urine
and 10% in faeces. Of the 80% recovered in urine,
approximately 38% is excreted as unchanged ertapenem
and approximately 37% as the ring-opened metabolite. |
| | Ertapenem Preparation Products And Raw materials |
|