- Methoxyethene
-

- $100.00 / 1KG
-
2023-09-08
- CAS:107-25-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
- Methoxyethene
-

- $0.00 / 1g
-
2022-05-12
- CAS:107-25-5
- Min. Order: 10g
- Purity: 99.0%
- Supply Ability: 10tons
- Methoxyethene
-

- $0.00 / 1KG
-
2022-05-10
- CAS:107-25-5
- Min. Order: 0.01KG
- Purity: 99%
- Supply Ability: 1 tons
|
| | Methoxyethene Basic information |
| Product Name: | Methoxyethene | | Synonyms: | CH2=CHOCH3;Vinyl methyl ether,inhibited;Vinl methyl ether,inhibited;methylvinyloxide;Metoxyethylene;Methyl vinyl ether(DownstreaM Products Cooperation Wanted);Methyl Vinyl Ether ( Vinyl Methyl Ether );methoxyethene | | CAS: | 107-25-5 | | MF: | C3H6O | | MW: | 58.08 | | EINECS: | 203-475-4 | | Product Categories: | | | Mol File: | 107-25-5.mol | 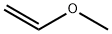 |
| | Methoxyethene Chemical Properties |
| | Methoxyethene Usage And Synthesis |
| Description |
Methoxyethene (MVE) is a colorless and flammable gas with a sweet and pleasant odor at room temperature. It is heavier than air and can diffuse to a relatively distant place at a lower place. MVE is soluble in ethanol, ether and acetone, but it is only slightly soluble in water. MVE is easily polymerized, so potassium hydroxide is often added as a polymerization inhibitor in the finished product.
| | Chemical Properties | Colorless compressed gas, or colorless
liquid. Slightly soluble in water; soluble in alcohol
and ether; easily polymerized. Commercial
material contains a small proportion of polymerization
inhibitor. | | Uses | Methyl Vinyl Ether is a reagent in the preparation of sodium alginate interpolymer complexes, pH-tunable drug carries. | | Uses | Copolymers used in coatings and lacquers;
modifier for alkyl, polystyrene, and ionomer resins;
plasticizer for nitrocellulose and adhesives. | | General Description | A colorless gas with a sweet odor. Shipped as a liquefied gas under own vapor pressure. Contact with the liquid may cause frostbite. Easily ignited. Vapors are heavier than air. Leaks may either be liquid or vapor. May asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat containers may rupture violently and rocket. | | Air & Water Reactions | Highly flammable. Reacts slowly with water to form acetaldehyde, reaction is not hazardous unless water is hot or acids are present. Form dangerous peroxides when exposed to air. | | Reactivity Profile | Methoxyethene reacts vigorously with oxidizing materials. Explosive in the form of vapor when exposed to heat, flame or strong oxidizing agents. Reacts, possibly explosively with halogens (bromine, chlorine) or hydrogen halides (hydrogen bromide, hydrogen chloride) [Baker, 1980, p. 487]. On contact with dilute acids or even mildly acidic solids (calcium chloride, ceramics) undergoes rapid, exothermic homopolymerization, which cannot be prevented by antioxidants. Must be stored in the presence of base [MVE Brochure, Billingham, ICI, 1962]. | | Health Hazard | Inhalation causes intoxication, blurring of vision, headache, dizziness, excitation, loss of consciousness. Liquid or concentrated vapor irritates eyes and causes frostbite of skin. Aspiration of the liquid will cause chemical pneumonitis. | | Fire Hazard | Behavior in Fire: Containers may explode. Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. | | Flammability and Explosibility | Extremely flammable | | Safety Profile | Mildly toxic by
ingestion. A very dangerous fire hazard
when exposed to heat, flame, or oxiduers.
Explosive in the form of vapor when
exposed to heat or flame. Can react
vigorously with oxidizing materials. To fight
fire, stop flow of gas. Potentially explosive
reaction with halogens (e.g., bromine,
chlorine) or hydrogen halides (e.g., hydrogen
bromide, hydrogen chloride). Reaction with
acids forms acetaldehyde. Weak acids
catalyze the exothermic polymerization of
the ether. The unstabilized ether can form
dangerous peroxides. When heated to
decomposition it emits acrid smoke and
irritating fumes. | | Synthesis | The industrial processes of MVE production include the acetylene route (Reppe process) and the acetal route according to the starting materials.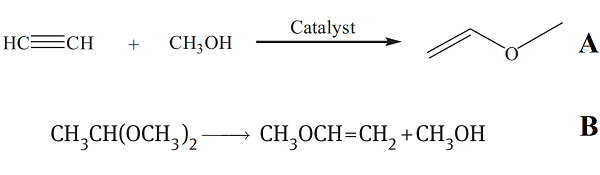 A. Acetylene undergoes a nucleophilic addition reaction with methanol in the presence of a strong base to produce MVE. The original Reppe process was carried out at a temperature of 160–165 °C under a 2–2.2 MPa pressure. The most commonly used catalyst for this reaction was an alkali metal hydroxide or an alkali metal alkoxide. Acetylene was usually diluted by nitrogen. However, this process requires high temperature and high pressure, leading to a safety problem in handling acetylene gas under such conditions. Another method is an equimolar mixture of acetylene gas and vaporized methanol, which is introduced into a fixed-bed reactor filled with a ZnO/SiO2 catalyst. The reaction temperature was in the range of 200 to 270 °C. MVE is the main product, and a significant amount of by-product (dimethoxyethane) was also generated by an equilibrium reaction of MVE and methanol to dimethoxyethane. A. Acetylene undergoes a nucleophilic addition reaction with methanol in the presence of a strong base to produce MVE. The original Reppe process was carried out at a temperature of 160–165 °C under a 2–2.2 MPa pressure. The most commonly used catalyst for this reaction was an alkali metal hydroxide or an alkali metal alkoxide. Acetylene was usually diluted by nitrogen. However, this process requires high temperature and high pressure, leading to a safety problem in handling acetylene gas under such conditions. Another method is an equimolar mixture of acetylene gas and vaporized methanol, which is introduced into a fixed-bed reactor filled with a ZnO/SiO2 catalyst. The reaction temperature was in the range of 200 to 270 °C. MVE is the main product, and a significant amount of by-product (dimethoxyethane) was also generated by an equilibrium reaction of MVE and methanol to dimethoxyethane.
B. MVE is prepared by pyrolysis of dimethyl acetal at high temperature in the presence of a solid catalyst. In general, the pyrolysis of dimethylacetal is carried out in the vapor phase. Dimethylacetal was vaporized and introduced into a fixed-bed reactor filled with calcium phosphate catalyst. In the reactor, dimethylacetal split into MVE and methanol at 320 °C and under atmospheric pressure. The conversion of dimethylacetal was 95%, and the selectivity to MVE was more than 98%. |
| | Methoxyethene Preparation Products And Raw materials |
|