|
|
| | Beryllium carbide Basic information |
| Product Name: | Beryllium carbide | | Synonyms: | beryllium acetylide;beryllium carbide;1,3-Diberyllapropadiene;Methanetetrayldiberyllium;Beryllium carbide (be2C);Einecs 208-050-7;Beryllium dicarbide | | CAS: | 506-66-1 | | MF: | C2Be | | MW: | 33.033582 | | EINECS: | 208-050-7 | | Product Categories: | | | Mol File: | 506-66-1.mol | 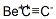 |
| | Beryllium carbide Chemical Properties |
| Melting point | decomposes at >2100℃ [MER06] | | density | 1.90 | | solubility | reacts with H2O | | form | red cubic crystals | | color | red cubic crystals, crystalline | | Water Solubility | decomposed very slowly by H2O; hydrolysis yields methane and beryllium hydroxide [KIR78] [MER06] | | Crystal Structure | Cubic | | CAS DataBase Reference | 506-66-1 | | EPA Substance Registry System | Beryllium carbide (Be2C) (506-66-1) |
| RIDADR | 1566 | | HazardClass | 6.1(b) | | PackingGroup | III |
| | Beryllium carbide Usage And Synthesis |
| Chemical Properties | Red cubic crystal; hard and refractory; density 1.90 g/cm3; decomposes when heated above 2,100°C; reacts with water. | | Physical properties | Brick-red or yellowish-red
octahedra. Uses: Nuclear reactor
cores. | | Uses | Beryllium acetylide is used in a nuclear reactor as core material,Nuclear reactor core material: Schwartz. | | Uses | Nuclear reactor core material: Schwartz, US 3170812 (1965 to USAEC). | | Uses | Beryllium carbide (Be2C) is used for the cores in nuclear reactors. | | Preparation | Beryllium carbide is prepared by heating the elements beryllium and carbon at elevated temperatures (above 900°C). It also may be prepared by reduction of beryllium oxide with carbon at a temperature above 1,500°C:
2BeO + 3C → (1500℃)→ Be2C + 2CO
Beryllium carbide decomposes very slowly in water:
Be2C + 2H2O → 2BeO + CH4
The rate of decomposition is faster in mineral acids with evolution of methane. However, in hot concentrated alkalies the reaction is very rapid, forming alkali metal beryllate and methane:
Be2C + 4NaOH → 2Na2BeO2 + CH4 |
| | Beryllium carbide Preparation Products And Raw materials |
|