|
|
| | Cobalt(II) acetate tetrahydrate Basic information |
| Product Name: | Cobalt(II) acetate tetrahydrate | | Synonyms: | COBALT ACETAT;Cobalt(II) acetate tetrahydrate, 99.999% metals basis;Aceticacid,cobalt(2+)salt,tetrahydrate;Cobaltous acetate Bis(acetato)tetraquocobalt;COBALT(II) ACETATE TETRAHYDRATE 98+% &;COBALT(II) ACETATE TETRAHYDRATE, 98+%, A .C.S. REAGENT;COBALT(II) ACETATE TETRAHYDRATE REAGEN&;COBALTOUS ACETATE TETRAHYDRATE, CRYSTALL IZED | | CAS: | 6147-53-1 | | MF: | C4H14CoO8 | | MW: | 249.08 | | EINECS: | 612-153-6 | | Product Categories: | cobalt , acetate;metal acetate salt;Inorganic Chemicals | | Mol File: | 6147-53-1.mol | 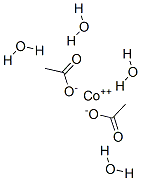 |
| | Cobalt(II) acetate tetrahydrate Chemical Properties |
| Melting point | 140 °C | | density | 1,71 g/cm3 | | refractive index | 1.542 | | storage temp. | Store at +5°C to +30°C. | | solubility | 348g/l | | form | Solid | | color | Red | | Specific Gravity | 1.71 | | Odor | Acetic acid odour | | PH Range | 6.8 | | PH | 7.2 (50g/l, H2O, 20℃) | | Water Solubility | 380 g/L (20 ºC) | | Sensitive | Hygroscopic | | Merck | 14,2433 | | BRN | 3731397 | | Stability: | Stable. Avoid moisture, heat. | | InChIKey | ZBYYWKJVSFHYJL-UHFFFAOYSA-L | | CAS DataBase Reference | 6147-53-1(CAS DataBase Reference) | | EPA Substance Registry System | Cobalt acetate tetrahydrate (6147-53-1) |
| | Cobalt(II) acetate tetrahydrate Usage And Synthesis |
| Chemical Properties | Reddish violet deliequecent crystals. | | Uses |
- Cobalt(II) acetate tetrahydrate is used to prepare complexes for examination of the properties of metals with unusual coordination geometries.
- used as a catalyst for oxidation and esterification.
- used as an industrial catalyst to harden paints and varnishes, an active catalyst for oxidation and esterification reactions.
| | Uses | Cobalt(II) acetate tetrahydrate is used as an industrial catalyst to harden paints and varnishes, an active catalyst for oxidation and esterification reactions. It is also used to prepare metal complexes having unusual coordination geometry. | | Uses | Cobalt(II) acetate tetrahydrate may be used in the preparation of [Co3(OH)2(H2O)2(aptet)4] (aptet = 4-aminophenyltetrazolate) and new tetradentate cobalt(II)-Schiff base complex. | | Application | Cobalt(II) acetate tetrahydrate may be used in the synthesis of the following:
- cobalt nanoparticles.
- cobalt(II)–aminophenyltetrazolate coordination polymer.
- tricobalt complexes with mixed μ-acetato and μ-pyrazolato ligands.
| | General Description | Cobalt(II) acetate tetrahydrate has an octahedral structure, the central cobalt orbit is coordinated by four water molecules and two acetate ligands. Cobalt(II) acetate tetrahydrate becomes anhydrous by 140oC. | | Safety Profile | Moderately toxic by
ingestion. Questionable carcinogen. A skin
and eye irritant. Human mutation data
reported. See also COBUT
COMPOUNDS. When heated to
decomposition it emits acrid smoke and
irritating fumes. | | Purification Methods | Several recrystallisations from 50% aqueous acetic acid give the tetrahydrate. It is converted to the anhydrous salt by drying at 80o/1mm for 60hours. [Beilstein 2 IV 120.] |
| | Cobalt(II) acetate tetrahydrate Preparation Products And Raw materials |
|