- Silver iodide
-

- $1.00 / 1KG
-
2024-04-25
- CAS:7783-96-2
- Min. Order: 1KG
- Purity: 99.91%
- Supply Ability: 200000
- Silver iodide
-

- $6.00 / 1KG
-
2024-04-20
- CAS:7783-96-2
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 2000KG/MONTH
- Silver iodide
-
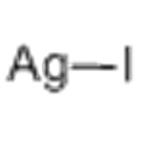
- $5.00 / 1KG
-
2024-04-02
- CAS:7783-96-2
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available
|
| | Silver iodide Chemical Properties |
| Melting point | 557°C | | Boiling point | 1506°C | | density | 5.68 g/mL at 25 °C (lit.) | | RTECS | VW4450000 | | solubility | insoluble in acid solutions | | form | Solid | | Specific Gravity | 6.01 | | color | Yellow | | Odor | odorless | | Water Solubility | 0.03 mg/L | | Sensitive | Light Sensitive | | Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m | | Merck | 14,8516 | | Solubility Product Constant (Ksp) | pKsp: 16.07 | | Exposure limits | ACGIH: TWA 0.01 ppm | | Stability: | Stability Light-sensitive. Incompatible with strong oxidizing agents. | | CAS DataBase Reference | 7783-96-2(CAS DataBase Reference) | | NIST Chemistry Reference | Silver iodide(7783-96-2) | | EPA Substance Registry System | Silver iodide (AgI) (7783-96-2) |
| | Silver iodide Usage And Synthesis |
| Description | Silver iodide (AgI) precipitates as a yellow, odor- and tasteless solid. Silver iodide acts as a very effective nucleus for the formation of ice crystals. Silver iodide has an important advantage over mercury as a subject for study of electrochemical properties of interfaces. It is also used as a solid lubricant for power contacts. It is also used as a local antiseptic.
| | References | [1] B. Vonnegut, The Nucleation of Ice Formation by Silver Iodide, Journal of Applied Physics, 1947, vol. 18, 593
[2] J. Lyklema and J. Th. G. Overbeek, Electrochemistry of silver iodide the capacity of the double layer at the silver iodide-water interface, Journal of Colloid Science, 1961, col. 16, 595-608
[3] Sylva Arnell and Gunnar Andersson, Silver Iodide as a Solid Lubricant for Power Contacts, Proceedings of the Forty-Seventh IEEE Holm Conference on Electrical Contacts, 2001
| | Chemical Properties | Yellow Crystalline Powder | | Physical properties | Light yellow hexagonal crystals or powder; darkens on exposure to light; density 5.68 g/cm3 ; melts at 558°C; vaporizes at 1,506°C; insoluble in water, most acids and ammonium carbonate solution; moderately soluble in concentrated solutions of alkali chloride, bromide, and thiosulfate; readily soluble in solutions of alkali cyanides, iodides and in hot concentrated hydriodic acid. | | Uses | Silver iodide is a yellow powder formed by the combination
of a soluble iodide combined with silver nitrate. Silver
iodide could also be formed by exposing metallic silver to the
fumes of bromine as in the daguerreotype process. This was
the primary halide used for all of the 19th century camera
processes until the introduction of the silver bromide gelatin
plate. With the exception of the daguerreotype, all silver iodide
processes relied on physical development using a reducing
agent such as gallic acid, pyrogallic acid, or ferrous sulfate, an
acid restrainer, and excess silver. | | Uses | Used in medicine for its caustic, astringent and antiseptic effects. Used in photography, and in the estimation of silver. Through heterogeneous nucleation, it is used in cloud seeding, relying on its beta-AgI crystal structure being similar to that of Ice. | | Uses | In cloud precipitation (rain-making). | | Preparation | Silver iodide is prepared by adding a solution of sodium or potassium iodide to a hot solution of silver nitrate: Ag+ (aq) + Iˉ (aq) → Ag I (s) The precipitate is washed with boiling water. The preparation is done in the dark under ruby red light. | | Definition | T3DB: Silver iodide is an iodide of silver. It is a photosensitive solid used in photography, as an antiseptic in medicine, and in rainmaking. Silver is a metallic element with the chemical symbol Ag and atomic number 47. It occurs naturally in its pure, free form, as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. | | Hazard | Silver iodide may cause irritation and psoriasis, and the main hazards encountered in the use and handling of silver iodide arise from its toxicological properties. It is toxic by a variety of routes (i.e. inhalation, ingestion and dermal contact) and exposures may include rashes, conjunctivitis, psoriasis (permanent greyish-grey discolouration of the skin, conjunctiva, and internal organs), headache, fever, hypersensitivity reactions, laryngitis and bronchitis. | | Health effects | Silver itself is not toxic to humans, but most silver salts are. In large doses, silver and compounds containing it can be absorbed into the circulatory system and become deposited in various body tissues, leading to argyria, which results in a blue-grayish pigmentation of the skin, eyes, and mucous membranes. Argyria is rare, and although, so far as known, this condition does not otherwise harm a person's health, it is disfiguring and usually permanent. Mild forms of argyria are sometimes mistaken for cyanosis. | | Flammability and Explosibility | Non flammable |
| | Silver iodide Preparation Products And Raw materials |
|